Abstract
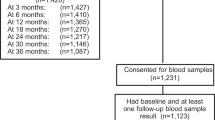
Chemotherapy-related amenorrhea (CRA) is associated with infertility and menopausal symptoms. Learning how frequently paclitaxel and trastuzumab cause amenorrhea is important. Most other adjuvant breast cancer therapies induce CRA in approximately 50 % of all premenopausal recipients [1]. 410 patients enrolled on the APT Trial, a single-arm phase 2 adjuvant study of 12 weeks of paclitaxel and trastuzumab followed by nine months of trastuzumab monotherapy. Eligible patients had ≤3 cm node-negative HER2 + breast cancers. Premenopausal enrollees were asked to complete menstrual surveys every 3–12 months for 72 months. Women who responded to at least one survey at least 15 months after chemotherapy initiation (and who did not undergo hysterectomy and/or bilateral oophorectomy or receive ovarian suppressing medications prior to 15 months) were included in this analysis. A participant was defined as having amenorrhea in follow-up if her self-reported last menstrual period at last follow-up was greater than 12 months prior to the survey. Among the 64 women in the evaluable population (median age at study entry 44 years, range 27–52 years), the median time between chemotherapy initiation and last menstrual survey was 51 months (range 16–79). 18 of 64 women (28 %, 95 % CI 18–41 %) were amenorrheic at that time point. Amenorrhea rates among premenopausal women treated with adjuvant paclitaxel and trastuzumab for early stage breast cancer appear lower than those seen historically with standard alkylator-based breast cancer regimens. Future studies are needed to understand the impact of this regimen on related issues of fertility and menopausal symptoms.


Similar content being viewed by others
References
Abusief ME, Missmer SA, Ginsburg ES, Weeks JC, Partridge AH (2010) The effects of paclitaxel, dose density, and trastuzumab on treatment-related amenorrhea in premenopausal women with breast cancer. Cancer 116(4):791–798. doi:10.1002/cncr.24835
Swain SM, Jeong JH, Wolmark N (2010) Amenorrhea from breast cancer therapy—not a matter of dose. N Engl J Med 363(23):2268–2270. doi:10.1056/NEJMc1009616
Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff J, Vogel VG, Erban JK, Rastogi P, Livingston RB, Perez EA, Mamounas EP, Land SR, Ganz PA, Wolmark N (2010) Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med 362(22):2053–2065. doi:10.1056/NEJMoa0909638
Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, Sukumvanich P (2006) Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol 24(7):1045–1051. doi:10.1200/JCO.2005.03.3969
Partridge A, Gelber S, Gelber RD, Castiglione-Gertsch M, Goldhirsch A, Winer E (2007) Age of menopause among women who remain premenopausal following treatment for early breast cancer: long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer 43(11):1646–1653. doi:10.1016/j.ejca.2007.04.006
Najafi S, Djavid GE, Mehrdad N, Rajaii E, Alavi N, Olfatbakhsh A, Najafi M, Bahrami A, Heidari K (2011) Taxane-based regimens as a risk factor for chemotherapy-induced amenorrhea. Menopause 18(2):208–212. doi:10.1097/gme.0b013e3181f3e6e7
Tham YL, Sexton K, Weiss H, Elledge R, Friedman LC, Kramer R (2007) The rates of chemotherapy-induced amenorrhea in patients treated with adjuvant doxorubicin and cyclophosphamide followed by a taxane. Am J Clin Oncol 30(2):126–132. doi:10.1097/01.coc.0000251398.57630.4f
Okanami Y, Ito Y, Watanabe C, Iijima K, Iwase T, Tokudome N, Takahashi S, Hatake K (2011) Incidence of chemotherapy-induced amenorrhea in premenopausal patients with breast cancer following adjuvant anthracycline and taxane. Breast Cancer 18(3):182–188. doi:10.1007/s12282-011-0256-7
Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA (2006) The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 21(10):2583–2592. doi:10.1093/humrep/del201
Fornier MN, Modi S, Panageas KS, Norton L, Hudis C (2005) Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer 104(8):1575–1579. doi:10.1002/cncr.21385
Davis AL, Klitus M, Mintzer DM (2005) Chemotherapy-induced amenorrhea from adjuvant breast cancer treatment: the effect of the addition of taxanes. Clin Breast Cancer 6(5):421–424. doi:10.3816/CBC.2005.n.046
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis M, Shapira I, Wolff AC, Carey LA, Overmoyer BA, Partridge AH, Guo H, Hudis CA, Krop IE, Burstein HJ, Winer EP (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372(2):134–141. doi:10.1056/NEJMoa1406281
Goodwin PJ, Ennis M, Pritchard KI, Trudeau M, Hood N (1999) Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17(8):2365–2370
Castiglione-Gertsch M, O’Neill A, Price KN, Goldhirsch A, Coates AS, Colleoni M, Nasi ML, Bonetti M, Gelber RD (2003) Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst 95(24):1833–1846
Walshe JM, Denduluri N, Swain SM (2006) Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol 24(36):5769–5779. doi:10.1200/JCO.2006.07.2793
Han HS, Ro J, Lee KS, Nam BH, Seo JA, Lee DH, Lee H, Lee ES, Kang HS, Kim SW (2009) Analysis of chemotherapy-induced amenorrhea rates by three different anthracycline and taxane containing regimens for early breast cancer. Breast Cancer Res Treat 115(2):335–342. doi:10.1007/s10549-008-0071-9
Anders C, Marcom PK, Peterson B, Gu L, Unruhe S, Welch R, Lyons P, Behera M, Copland S, Kimmick G, Shaw H, Snyder S, Antenos M, Woodruff T, Blackwell K (2008) A pilot study of predictive markers of chemotherapy-related amenorrhea among premenopausal women with early stage breast cancer. Cancer Invest 26(3):286–295. doi:10.1080/07357900701829777
Mourits MJ, de Vries EG, ten Hoor KA, van der Zee AG, Willemse PH (2007) Beware of amenorrhea during tamoxifen: it may be a wolf in sheep’s clothing. J Clin Oncol 25(24):3787–3788. doi:10.1200/JCO.2007.11.1633 (author reply 3788–3789)
Sukumvanich P, Case LD, Van Zee K, Singletary SE, Paskett ED, Petrek JA, Naftalis E, Naughton MJ (2010) Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer 116(13):3102–3111. doi:10.1002/cncr.25106
Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA, Burstein HJ, Martino S, Davidson NE, Geyer CE Jr, Walley BA, Coleman R, Kerbrat P, Buchholz S, Ingle JN, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Colleoni M, Viale G, Coates AS, Goldhirsch A, Gelber RD (2015) Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 372(5):436–446. doi:10.1056/NEJMoa1412379
Bernhard J, Zahrieh D, Castiglione-Gertsch M, Hurny C, Gelber RD, Forbes JF, Murray E, Collins J, Aebi S, Thurlimann B, Price KN, Goldhirsch A, Coates AS (2007) Adjuvant chemotherapy followed by goserelin compared with either modality alone: the impact on amenorrhea, hot flashes, and quality of life in premenopausal patients—the International Breast Cancer Study Group Trial VIII. J Clin Oncol 25(3):263–270. doi:10.1200/JCO.2005.04.5393
Anderson RA, Cameron DA (2011) Pretreatment serum anti-mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab 96(5):1336–1343. doi:10.1210/jc.2010-2582
Minton SE, Munster PN (2002) Chemotherapy-induced amenorrhea and fertility in women undergoing adjuvant treatment for breast cancer. Cancer Control 9(6):466–472
Acknowledgments
This trial was supported by Genentech. This publication was made possible by CTSA Grant Numbers UL1TR000135 and KL2TR000136-09 (KJR) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Precis: Paclitaxel–trastuzumab is likely less gonadotoxic than standard chemotherapy for breast cancer. This regimen may be appealing for women who wish to preserve ovarian function.
Conflict of interest
Authors Ruddy, Guo, Barry, Moy, Marcom, Shapira, Carey, Overmoyer, Hudis, Burstein, and Partridge have no conflicts of interest to disclose. Authors Dang, Rugo, Wolff, Krop, Winer, and Tolaney have received funding from Roche/Genentech. Author Yardley discloses that she has served in a consultant/advisory role with Genentech. Author Albain also has served on ad hoc advisory boards with Roche/Genentech, unrelated to this study. Author Ellis has received remuneration from Pfiser, AstraZeneca, Novartis, and Celgene. He has also held a consultant/advisory role with Nanostring and owns Bioclassifier, LLC stock.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruddy, K.J., Guo, H., Barry, W. et al. Chemotherapy-related amenorrhea after adjuvant paclitaxel–trastuzumab (APT trial). Breast Cancer Res Treat 151, 589–596 (2015). https://doi.org/10.1007/s10549-015-3426-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3426-z