Abstract
Objectives
Sarcopenia’s temporal profile can be regarded as a dynamic process with distinct states, in which malnutrition plays an important role. This study aimed to address two research gaps: sarcopenia’s transitional dynamics and associations of nutritional indices with state transitions in community-dwelling Chinese adults aged 50 and older.
Design
A prospective population-based cohort study.
Setting
Community-based setting in western China.
Participants
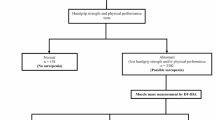
The analytic sample included data from 1910 participants aged ≥ 50 in the West China Health and Aging Trend study between 2018–2022.
Measurements
We defined three states: the initial normal state (normal muscle strength, physical performance and muscle mass), the worst sarcopenia state (low muscle mass plus low muscle strength and/or low physical performance) and the intermediate subclinical state (the other scenarios). The relevant measurement methods and cut-off points were based on the 2019 AWGS consensus. Using a continuous-time multistate Markov model, we calculated probabilities of transitions between different states over 1, 2 and 4 years; we also examined associations between nutritional indices and transitions, including body mass index (BMI), calf circumference (CC), mid-arm circumference (MAC), triceps skinfold thickness (TST), albumin (ALB), geriatric nutrition risk index (GNRI), vitamin D (VitD) and prealbumin (PA).
Results
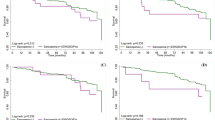
For individuals in the normal state, their probabilities of remaining stable versus progressing to a subclinical state were 53.4% versus 42.1% at 2 years, and 40.6% versus 49.0% at 4 years. In the subclinical population, their 2- and 4-year chances were 60.2% and 51.2% for maintaining this state, 11.8% and 16.2% for developing sarcopenia, 28.0% and 32.6% for reverting to normal. For sarcopenic individuals, the likelihood of staying stable versus retrogressing to the subclinical state were 67.0% versus 26.3% at 2 years, and 48.3% versus 36.3% at 4 years. Increased BMI, CC, MAC, TST, ALB, GNRI and PA correlated with reversion from the subclinical state, among which increased TST, ALB and PA were also paralleled with reversion from sarcopenia, while decreased BMI, CC, MAC, TST and GNRI were associated with progression to sarcopenia. VitD was not significantly associated with any transitions.
Conclusion
This study reveals how sarcopenia changes over time in a Chinese population. It also highlights the usefulness of simple and cost-effective nutritional status indices for indicating state transitions, which can help identify individuals at risk of sarcopenia and guide targeted interventions within the optimal time window.


Similar content being viewed by others
References
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet (London, England). Jun 29 2019;393(10191):2636–2646. doi:https://doi.org/10.1016/s0140-6736(19)31138-9
Petermann-Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. Feb 2022;13(1):86–99. doi:https://doi.org/10.1002/jcsm.12783
Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PloS one. 2017;12(1):e0169548. doi:https://doi.org/10.1371/journal.pone.0169548
Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc. Mar 2020;21(3):300–307.e2. doi:https://doi.org/10.1016/j.jamda.2019.12.012
Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. Jun 2019;10(3):485–500. doi:https://doi.org/10.1002/jcsm.12411
Kim H, Suzuki T, Kim M, et al. Incidence and predictors of sarcopenia onset in community-dwelling elderly Japanese women: 4-year follow-up study. J Am Med Dir Assoc. Jan 2015;16(1):85.e1–8. doi:https://doi.org/10.1016/j.jamda.2014.10.006
Qaisar R, Karim A, Muhammad T, Shah I, Khan J. Prediction of sarcopenia using a battery of circulating biomarkers. Scientific reports. Apr 21 2021;11(1):8632. doi:https://doi.org/10.1038/s41598-021-87974-6
Ágnes T, Vishal K, Girish N. Regression model for the prediction of risk of sarcopenia among older adults. Muscle Ligaments and Tendons Journal. 2019
Murphy RA, Ip EH, Zhang Q, et al. Transition to sarcopenia and determinants of transitions in older adults: a population-based study. The journals of gerontology Series A, Biological sciences and medical sciences. Jun 2014;69(6):751–8. doi:https://doi.org/10.1093/gerona/glt131
Trevisan C, Vetrano DL, Calvani R, Picca A, Welmer AK. Twelve-year sarcopenia trajectories in older adults: results from a population-based study. J Cachexia Sarcopenia Muscle. Feb 2022;13(1):254–263. doi:https://doi.org/10.1002/jcsm.12875
Sieber CC. Malnutrition and sarcopenia. Aging Clin Exp Res. Jun 2019;31(6):793–798. doi:https://doi.org/10.1007/s40520-019-01170-1
Xiang Q, Li Y, Xia X, et al. Associations of geriatric nutrition risk index and other nutritional risk-related indexes with sarcopenia presence and their value in sarcopenia diagnosis. BMC Geriatr. Apr 15 2022;22(1):327. doi:https://doi.org/10.1186/s12877-022-03036-0
Keller U. Nutritional Laboratory Markers in Malnutrition. Journal of clinical medicine. May 31 2019;8(6)doi:https://doi.org/10.3390/jcm8060775
Beck FK, Rosenthal TC. Prealbumin: a marker for nutritional evaluation. Am Fam Physician. Apr 15 2002;65(8):1575–8.
Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients. Nov 21 2019;11(12)doi:https://doi.org/10.3390/nu11122861
Hirani V, Cumming RG, Naganathan V, et al. Longitudinal Associations Between Vitamin D Metabolites and Sarcopenia in Older Australian men: The Concord Health and Aging in Men Project. The journals of gerontology Series A, Biological sciences and medical sciences. Dec 12 2017;73(1):131–138. doi:https://doi.org/10.1093/gerona/glx086
Chen Q, Hao Q, Ding Y, Dong B. The Association between Sarcopenia and Prealbumin Levels among Elderly Chinese Inpatients. J Nutr Health Aging. 2019;23(2):122–127. doi:https://doi.org/10.1007/s12603-018-1130-5
Ingenbleek Y. Plasma Transthyretin as A Biomarker of Sarcopenia in Elderly Subjects. Nutrients. Apr 21 2019;11(4)doi:https://doi.org/10.3390/nu11040895
Hou L, Liu X, Zhang Y, et al. Cohort Profile: West China Health and Aging Trend (WCHAT). J Nutr Health Aging. 2021;25(3):302–310. doi:https://doi.org/10.1007/s12603-020-1530-1
Bouillanne O, Morineau G, Dupont C, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. The American journal of clinical nutrition. Oct 2005;82(4):777–83. doi:https://doi.org/10.1093/ajcn/82.4.777
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. Autumn 1969;9(3):179–86.
Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. Journal of the American Geriatrics Society. Dec 1983;31(12):721–7. doi:https://doi.org/10.1111/j.1532-5415.1983.tb03391.x
Mahoney FI, Barthel DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. Feb 1965;14:61–5.
Graf C. The Lawton instrumental activities of daily living scale. Am J Nurs. Apr 2008;108(4):52–62; quiz 62–3. doi:https://doi.org/10.1097/01.Naj.0000314810.46029.74
Wang YY, Deng CY, Ding D, et al. [Development and validation of the China Leisure Time Physical Activity Questionnaire in the elderly]. Practical Geriatrics. 2019;33(03):229–233 (in Chinese).
Conway JM, Irwin ML, Ainsworth BE. Estimating energy expenditure from the Minnesota Leisure Time Physical Activity and Tecumseh Occupational Activity questionnaires - a doubly labeled water validation. Journal of clinical epidemiology. Apr 2002;55(4):392–9. doi:https://doi.org/10.1016/s0895-4356(01)00497-8
Ge M, Zhang Y, Zhao W, et al. Prevalence and Its Associated Factors of Physical Frailty and Cognitive Impairment: Findings from the West China Health and Aging Trend Study (WCHAT). J Nutr Health Aging. 2020;24(5):525–533. doi:https://doi.org/10.1007/s12603-020-1363-y
Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society. Oct 1975;23(10):433–41. doi:https://doi.org/10.1111/j.1532-5415.1975.tb00927.x
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine. May 22 2006;166(10):1092–7. doi:https://doi.org/10.1001/archinte.166.10.1092
Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. Oct 1999;14(10):858–65. doi:https://doi.org/10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8
Jackson C. Multi-State Models for Panel Data: The msm Package for R. 2011
Prasad N, Gupta A, Sinha A, et al. Confounding effect of comorbidities and malnutrition on survival of peritoneal dialysis patients. J Ren Nutr. Nov 2010;20(6):384–91. doi:https://doi.org/10.1053/j.jrn.2010.01.001
Gong G, Wan W, Zhang X, Liu Y, Liu X, Yin J. Correlation between the Charlson comorbidity index and skeletal muscle mass/physical performance in hospitalized older people potentially suffering from sarcopenia. BMC Geriatr. Dec 23 2019;19(1):367. doi:https://doi.org/10.1186/s12877-019-1395-5
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. Jul 1 2019;48(4):601. doi:https://doi.org/10.1093/ageing/afz046
Liu B, Hu X, Zhang Q, et al. Usual walking speed and all-cause mortality risk in older people: A systematic review and meta-analysis. Gait Posture. Feb 2016;44:172–7. doi:https://doi.org/10.1016/j.gaitpost.2015.12.008
Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med. Dec 22 2016;14(1):215. doi:https://doi.org/10.1186/s12916-016-0763-7
Reijnierse EM, de van der Schueren MAE, Trappenburg MC, Doves M, Meskers CGM, Maier AB. Lack of knowledge and availability of diagnostic equipment could hinder the diagnosis of sarcopenia and its management. PloS one. 2017;12(10):e0185837. doi:https://doi.org/10.1371/journal.pone.0185837
Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep (Oxf). Nov 2016;4(4):272–280. doi:https://doi.org/10.1093/gastro/gow013
Kokot T, Malczyk E, Ziółko E, Muc-Wierzgoń M, Fatyga E. Chapter 9 - Assessment of Nutritional Status in the Elderly. In: Watson RR, ed. Nutrition and Functional Foods for Healthy Aging. Academic Press;2017:75–81.
Jones CH, Smye SW, Newstead CG, Will EJ, Davison AM. Extracellular fluid volume determined by bioelectric impedance and serum albumin in CAPD patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. Feb 1998;13(2):393–7. doi:https://doi.org/10.1093/oxfordjournals.ndt.a027836
Hao X, Li D, Zhang N. Geriatric Nutritional Risk Index as a predictor for mortality: a meta-analysis of observational studies. Nutr Res. Nov 2019;71:8–20. doi:https://doi.org/10.1016/j.nutres.2019.07.005
Gärtner S, Kraft M, Krüger J, et al. Geriatric nutritional risk index correlates with length of hospital stay and inflammatory markers in older inpatients. Clin Nutr. Aug 2017;36(4):1048–1053. doi:https://doi.org/10.1016/j.clnu.2016.06.019
Landi F, Camprubi-Robles M, Bear DE, et al. Muscle loss: The new malnutrition challenge in clinical practice. Clin Nutr. Oct 2019;38(5):2113–2120. doi:https://doi.org/10.1016/j.clnu.2018.11.021
Ziaaldini MM, Marzetti E, Picca A, Murlasits Z. Biochemical Pathways of Sarcopenia and Their Modulation by Physical Exercise: A Narrative Review. Frontiers in medicine. 2017;4:167. doi:https://doi.org/10.3389/fmed.2017.00167
Yang S, Wang S, Tai P, et al. Central and Peripheral Adiposity Had Different Effect on Disability in Centenarians. Frontiers in endocrinology. 2021;12:635205. doi:https://doi.org/10.3389/fendo.2021.635205
Dhana K, Koolhaas CM, Schoufour JD, et al. Association of anthropometric measures with fat and fat-free mass in the elderly: The Rotterdam study. Maturitas. Jun 2016;88:96–100. doi:https://doi.org/10.1016/j.maturitas.2016.03.018
Zhu Y, Lin Q, Zhang Y, et al. Mid-upper arm circumference as a simple tool for identifying central obesity and insulin resistance in type 2 diabetes. PloS one. 2020;15(5):e0231308. doi:https://doi.org/10.1371/journal.pone.0231308
Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. Nov 2008;88(11):1336–44. doi:https://doi.org/10.2522/ptj.20080079
Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. Journal of applied physiology (Bethesda, Md: 1985). Jun 2001;90(6):2157–65. doi:https://doi.org/10.1152/jappl.2001.90.6.2157
Ranasinghe RN, Biswas M, Vincent RP. Prealbumin: The clinical utility and analytical methodologies. Ann Clin Biochem. Jan 2022;59(1):7–14. doi:https://doi.org/10.1177/0004563220931885
Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10(2):e1001383. doi:https://doi.org/10.1371/journal.pmed.1001383
Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. The American journal of clinical nutrition. Aug 2002;76(2):473–81. doi:https://doi.org/10.1093/ajcn/76.2.473
Acknowledgement: We thank all the participants for their contribution in the WCHAT study.
Funding
Funding statement: This study was supported by the following grants: Chinese National Science & Technology Pillar Program (2020YFC2005600); Sichuan Science and Technology Program (2021YFS0136); 1.3.5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (19HXFH012); National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2023LC008, Z20191012); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21005); Project of Max Cynader Academy of Brain Workstation, WCHSCU (HXYS19005).
Author information
Authors and Affiliations
Contributions
Author Contribution: Yuxiao Li and Qiao Xiang contributed to the conception of the study, performed the data analyses, wrote the main manuscript text, as well as prepared the tables and figures. Birong Dong supervised the project and provided instructions on result interpretation. Rui Liang, Quhong Song and Linghui Deng contributed to data collection and database construction. Jirong Yue and Ni Ge supervised the project, provided instructions on the study design and revised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical statements: The research complied with the current laws of China. The study was approved by the Ethical Committee of Sichuan University West China Hospital and adhered to the principles of the Declaration of Helsinki.
Conflict of Interest: The authors declare that they have no conflicts of interest to this work.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, Y., Xiang, Q., Dong, B. et al. Transitional Dynamics of Sarcopenia and Associations of Nutritional Indices with State Transitions in Chinese aged ≥ 50. J Nutr Health Aging 27, 741–751 (2023). https://doi.org/10.1007/s12603-023-1974-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12603-023-1974-1