Abstract
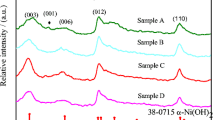
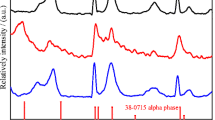
Nickel hydroxide is widely used as cathode materials in nickel-metal secondary batteries. In this work, Mn-substituted nickel hydroxide samples with a special α/β mixed phase structure were synthesized by chemical co-precipitation method. The physical properties were characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), differential scanning calorimetry (DSC) and field emission scanning electron microscopy (FE-SEM). The results show that the structure of the samples and the amount of intercalated anions and water molecules are highly related to the content of the Mn substituted. Their electrochemical performances were characterized by charge/discharge tests and electrochemical cycle tests. The results demonstrate that the Mn-substituted samples with a α/β mixed phase structure perform a much higher discharge capacity than normal β-nickel hydroxide. The specific discharge capacity reaches 330 mAh·g−1 after 50 cycles of charge/discharge in charging rate of 0.2C under ambient temperature. Meanwhile, the samples show no capacity loss in electrochemical cycles, which indicates that the mixed phase nickel hydroxide maintains high structure stability.






Similar content being viewed by others
References
Watanabe K, Kikuoka T. Physical and electrochemical characteristics of nickel hydroxide as a positive material for rechargeable alkaline batteries. J Appl Electrochem. 1995;25(3):219.
Wang DH, Luo YC, Yan RX, Zhang FL, Kang L. Phase structure and electrochemical properties of La0.67Mg0.33Ni3.0−xCox (x = 0, 0.25, 0.50, 0.75) hydrogen storage alloys. J Alloy Compd. 2006;413(1–2):193.
Bode H, Dehmelt K, Witte J. Nickel hydroxide electrodes. I. Nickel(II) hydroxide hydrate. Electrochem Acta. 1966;11:1079.
Delahaye-Vidal A, Beaudoin B, Sac-Epee N, Tekaia-Elhsissen K, Audemer A, Figlarz M. Structural and textural investigations of the nickel hydroxide electrode. Solid State Ion. 1996;84(3–4):239.
Van der Ven A, Morgan D, Meng YS, Ceder G. Phase stability of nickel hydroxides and oxyhydroxides. J Electrochem Soc. 2006;153(2):A210.
Li YW, Yao JH, Liu CJ, Zhao WM, Deng WX, Zhong SK. Effect of interlayer anions on the electrochemical performance of Al-substituted α-type nickel hydroxide electrodes. Int J Hydrog Energy. 2010;35(6):2539.
Wu MY, Wang JM, Zhang JQ, Cao CN. Structure and electrochemical performance of Mn-substituted nickel hydroxide. Acta Phys Chim Sin. 2005;21(5):523.
Demourgues-Guerlou L, Delmas C. Structure and properties of precipitated nickel-iron hydroxides. J Power Sources. 1993;45(93):281.
Chen H, Wang JM, Pan T, Xiao HM, Zhang JQ, Cao CN. Effects of coprecipitated zinc on the structure and electrochemical performance of Ni/Al-layered double hydroxide. Int J Hydrog Energy. 2002;27(5):489.
Leng YJ, Liu B, Wang FJ, Zhou JX, Xiao Y, Ma ZY. Preparation, structure and electrochemical performance of aluminum-substituted nickel hydroxide. Chin J Power Sources. 2000;24(6):326.
Jung JH, Lee HH, Yu JS, Jang KJ, Lee JY. Self-discharge mechanism of vanadium–titanium metal hydride electrodes for Ni–MH rechargeable battery. Met Mater. 1997;3(3):178.
French HM, Henderson MJ, Hillman AR, Vieil E. Ion and solvent transfer discrimination at a nickel hydroxide film exposed to LiOH by combined electrochemical quartz crystal microbalance (EQCM) and probe beam deflection (PBD) techniques. J Electroanal Chem. 2001;500(1–2):192.
Li YW, Yao JH, Zhu YX, Zou ZG, Wang HB. Synthesis and electrochemical performance of mixed phase α/β nickel hydroxide. J Power Sources. 2012;203:177.
Ramesh TN, Vishnu Kamath P. Synthesis of nickel hydroxide: effect of precipitation conditions on phase selectivity and structural disorder. J Power Sources. 2006;156(2):655.
Yang CC. Synthesis and characterization of active materials of Ni(OH)2 powders. Int J Hydrog Energy. 2002;27(10):1071.
Li YX, Yang CZ, Lou YW, Xia BJ. Investigation of physical mechanism for electric conducting during charge-discharge process in MH/Ni battery. Acta Chim Sin. 2009;67(9):901.
Han ES, Kang HX, Wei ZH, Zhang HL. Study on the preparation and performance of multiphase nickel hydroxide. Chin J Power Sources. 2007;31(5):396.
Li QY, Wang RN, Nie ZR, Wei Q, Wang ZH. Preparation of three-dimensional flower-like Ni(OH)2 nanostructures by a facile template-free solution process. J Alloy Compd. 2010;496(1–2):300.
Chang ZR, Tang HW, Chen JG. Surface modification of spherical nickel hydroxide for nickel electrodes. Electrochem Commun. 1999;1(11):513.
Wang Y, Zhu QS. Electrochemical properties and controlled-synthesis of hierarchical β-Ni(OH)2 micro-flower and hollow microspheres. Mater Res Bull. 2010;45(12):1844.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21403015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lei, H., Pan, Y., Guo, RG. et al. Synthesis and electrochemical properties of Mn-substituted high-capacity nickel hydroxide. Rare Met. 41, 1977–1982 (2022). https://doi.org/10.1007/s12598-015-0514-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0514-5