Abstract
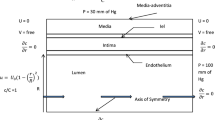
A two-dimensional (2D) numerical simulation of convective–diffusive transport of LDL in the artery wall, coupled with the wall shear stress gradient (WSSG)-dependent LDL consumption of smooth muscle cells (SMCs) is presented. SMCs are modeled as an array of solid cylindrical pillars embedded in a continuous porous media which represents the interstitial proteoglycan and collagen fiber matrix. The internal elastic lamina (IEL), which separates the artery media from the intima, is modeled as an impermeable barrier to both water and LDL except for the fenestral pores that are assumed to be uniformly distributed over the IEL. The predictions demonstrate a range of interesting features of LDL transport and uptake in the media. For cells immediately below the fenestral pores, LDL uptake of SMCs is highly dependent on WSSG. Moreover, the rate of LDL consumption by SMCs is also affected by the diameter of the fenestral pore. This will be helpful in understanding the involvement of transmural transport processes in the initiation and development of atherosclerosis.










Similar content being viewed by others
References
Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–9.
Schwenke DC, St Clair RW. Influx, efflux, and accumulation of LDL in normal arterial areas and atherosclerotic lesions of white carneau pigeons with naturally occurring and cholesterol-aggravated aortic atherosclerosis. Arterioscl Thromb Vas Biol. 1993;13:1368–81.
Han Y, Ganatos P, Weinbaum S. Transmission of steady and oscillatory fluid shear stress across epithelial and endothelial surface structures. Phys Fluids. 2005;17:031508.
van den Berg BM, Spaan JAE, Rolf TM, Vink H. Atherogenic region and diet diminish glycocalyx dimension and increase intima-to-media ratios at murine carotid artery bifurcation. Am J Physiol Heart C. 2006;290:H915–20.
Tada S, Tarbell JM. Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am J Physiol Heart C. 2000;278:H1589–97.
Yang N, Vafai K. Modeling of low-density lipoprotein (LDL) transport in the artery—effects of hypertension. Int J Heat Mass Transf. 2006;49:850–67.
Dabagh M, Jalali P, Konttinen YT. The study of wall deformation and flow distribution with transmural pressure by three-dimensional model of thoracic aorta wall. Med Eng Phys. 2009;31:816–24.
Yuan F, Chien S, Weinbaum S. A new view of convective–diffusive transport processes in the arterial intima. J Biomech Eng T ASME. 1991;113:314–29.
Wang DM, Tarbell JM. Modeling interstitial flow in an artery wall allows estimation of wall shear stress on smooth muscle cells. J Biomech Eng T AMSE. 1995;117:358–63.
Brinkman HC. A calculation of the viscous force exerted by a flowing fluid on a dense swarm of particles. Appl Sci Res A. 1947;1:27–34.
Bratzler RL, Chisolm GM, Colton CK, Smith KA, Lees RS. The distribution of labeled low-density lipoprotein across the rabbit thoracic aorta in vivo. Atherosclerosis. 1977;28:289–307.
Tedgui A, Lever MJ. Filtration through damaged and undamaged rabbit thoracic aorta. Am J Physiol Heart C. 1984;247:H784–91.
Weinbaum S, Ganatos P, Pfeffer R, Wen GB, Lee M, Chien S. On the time-dependent diffusion of macromolecules through transient open junctions and their subendothelial spread. I. Short-time model for cleft exit region. J Theor Biol. 1988;135:1–30.
Dardik A, Yamashita A, Aziz F, Asada H, Sumpio BE. Shear stress-stimulated endothelial cells induce smooth muscle cell chemotaxis via platelet-derived growth factor-BB and interleukin-1α. J Vasc Surg. 2005;41:321–31.
Gambillara V, Montorzi G, Haziza-Pigeon C, Stergiopulos N, Silacci P. Arterial wall response to ex vivo exposure to oscillatory shear stress. J Vasc Res. 2005;42:535–44.
Lei M, Kleinstreuer C, Truskey GA. A focal stress gradient-dependent mass transfer mechanism for atherogenesis in branching arteries. Med Eng Phys. 1996;18:326–32.
Huang ZJ, Tarbell JM. Numerical simulation of mass transfer in porous media of blood vessel walls. Am J Physiol Heart C. 1997;273:H464–77.
Tada S, Tarbell JM. Internal elastic lamina affects the distribution of macromolecules in the arterial wall—a computational study. Am J Physiol Heart C. 2004;287:H905–13.
Wang S, Tarbell JM. Effect of fluid flow on smooth muscle cells in a 3-dimensional collagen gel model. Arterioscl Thromb Vas Biol. 2000;20:2220–5.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tada, S., Ozono, H. Computational study of LDL mass transport in the artery wall. J Biorheol 25, 27–35 (2011). https://doi.org/10.1007/s12573-011-0034-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12573-011-0034-3