Abstract
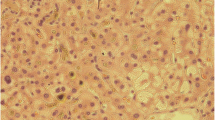
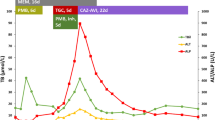
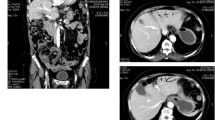
Trimethoprim/sulfamethoxazole is well known to cause intra-hepatic cholestasis which in rare instances can be prolonged and lead to vanishing bile duct syndrome. The risk regarding the potential for cross-reactivity between structurally related molecules such as dapsone and trimethoprim/sulfamethoxazole in causing hepatotoxicity is scarce. Herein, we report a case of vanishing bile duct syndrome following dapsone use in a patient with HIV infection and a recent history of trimethoprim/sulfamethoxazole-induced cholestasis. The patient had severe and protracted cholestasis during 2 years of follow-up and eventually died of liver failure.




Similar content being viewed by others
References
Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, 2016 [Internet]. 2016. https://www.cdc.gov/antibiotic-use/community/programs-measurement/state-local-activities/outpatient-antibiotic-prescriptions-US-2016.html. Accessed 20 Feb 2019.
White PL, Price JS, Backx M. Therapy and management of Pneumocystis jirovecii infection. J Fungi. 2018;4:127.
Chalasani NP, Reddy KRK, Fontana RJ, et al. Idiosyncratic drug induced liver injury in African–Americans is associated with greater morbidity and mortality compared to Caucasians. Am J Gastroenterol. 2017;112:1382–8.
Molleston JP, Fontana RJ, Lopez MJ, et al. Characteristics of idiosyncratic drug-induced liver injury in children: results from the DILIN prospective study. J Pediatr Gastroenterol Nutr. 2011;53:182–9.
Faria LC, Resende CC, Couto CA, et al. Severe and prolonged cholestasis caused by trimethoprim-sulfamethoxazole: a case report. Clinics. 2009;64:71–4.
Lin X, Garg S, Mattson CL, et al. Prescription of Pneumocystis jiroveci pneumonia prophylaxis in HIV-infected patients. J Int Assoc Provid AIDS Care. 2016;15:455–8.
Ogilvie LA, Toghill PJ. Cholestatic jaundice due to co-trimoxazole. Post Grad Med J. 1980;653:202–4.
Langlois M, Derk F, Belczyk R, et al. Trimethoprim-sulfamethoxazole-induced Stevens–Johnson syndrome: a case report. J Am Podiatr Med Assoc. 2010;100:299–303.
Hanses F, Zierhut S, Schölmerich J, et al. Severe and long lasting cholestasis after high-dose co-trimoxazole treatment for Pneumocystis pneumonia in HIV-infected patients-a report of two cases. Int J Infect Dis. 2009;13:467–9.
Cho HJ, Jwa HJ, Kim KS, et al. Urosodeoxycholic acid therapy in a child with trimethoprim-sulfamethoxazole induced vanishing bile duct syndrome. Pediatr Gastroenterol Hepatol Nutr. 2013;16:273–8.
Nakanuma Y, Tsuneyama K, Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. 2001;8:303–15.
Bonkovsky HL, Kleiner DE, Gu J, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77.
Zaman F, Ye G, Kd A, et al. Case report successful orthotopic liver transplantation after trimethoprim-sulfamethoxazole associated fulminant liver failure. Clin Transplant. 2003;17:461–4.
Kowdley KV, Keeffe EB, Fawaz KA. Prolonged cholestasis due to trimethoprim sulfamethoxazole. Gastroenterology. 1992;102:2148–50.
Quaresma MV, Bernardes Filho F, Hezel J, et al. Dapsone in the treatment of pemphigus vulgaris: adverse effects and its importance as a corticosteroid sparing agent. An Bras Dermatol. 2015;90:S51–4.
Tom De Roche LBS. The Day Dapsone DRESSed as DILI. J Clin Toxicol. 2015;05:1–2.
Beumont MG, Graziani A, Ubel PA, et al. Safety of dapsone as Pneumocystis carinii pneumonia prophylaxis in human immunodeficiency virus-infected patients with allergy to trimethoprim/sulfamethoxazole. Am J Med. 1996;100:611–6.
Holtzer CD, Flaherty JFJ, Coleman RL. Cross-reactivity in HIV-infected patients switched from trimethoprim-sulfamethoxazole to dapsone. Pharmacotherapy. 1998;18:831–5.
May SM, Motosue MS, Park MA. Dapsone is often tolerated in HIV-infected patients with history of sulfonamide antibiotic intolerance. J Allergy Clin Immunol Pract [Internet] Am Acad Allergy Asthma Immunol. 2017;5:831–3.
Reau NS, Jensen DM. Vanishing bile duct syndrome. Clin Liver Dis. 2008;12:203–17.
Aithal PG, Day CP. The natural history of histologically proved drug induced liver disease. Gut. 1999;44:731–5.
Acknowledgements
We would like to acknowledge that this work was funded in part by a Grant provided to Dr. Fontana by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to the University of Michigan (2U01-DK065184-06).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Fontana has received Grant support from Gilead, BristolMyersSquibb, and Abbvie and provided consultation to Sanofi. Dr. Mutchnick has research Grants from Proteus and Gilead and also a Member of Speakers Bureau for Gilead, Abbvie, Intercept and Chronic Liver Disease Foundation. Financial disclosures for the remaining authors: None.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patient for the case report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kathi, P.R., Tama, M., Ehrinpreis, M. et al. Vanishing bile duct syndrome arising in a patient with HIV infection sequentially treated with trimethoprim/sulfamethoxazole and dapsone. Clin J Gastroenterol 13, 276–280 (2020). https://doi.org/10.1007/s12328-019-01022-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-019-01022-5