Abstract
Background
Cefuroxime very rarely causes drug-induced liver injury. We present a case of a patient with paradoxical worsening of jaundice caused by cefuroxime-induced cholestasis following therapeutic endoscopic retrograde cholangiopancreatography for a distal common bile duct stone.
Case presentation
A 51-year-old, previously healthy Sri Lankan man presented to our hospital with obstructive jaundice caused by a distal common bile duct stone. Endoscopic retrograde cholangiopancreatography with stone extraction, common bile duct clearance, and stenting failed to improve the cholestasis, with paradoxical worsening of his jaundice. A liver biopsy revealed features of drug-induced intrahepatic cholestasis. Although his case was complicated by an episode of cholangitis, the patient made a complete recovery in 4 months with supportive treatment and withdrawal of the offending drug.
Conclusions
This case highlights a very rare drug-induced liver injury caused by cefuroxime as well as our approach to treating a patient with paradoxical worsening of jaundice after therapeutic endoscopic retrograde cholangiopancreatography.
Similar content being viewed by others
Background
Cefuroxime is a second-generation cephalosporin known to very rarely cause drug-induced liver injury (DILI). There have been fewer than five previously reported cases of cefuroxime causing DILI and cholestasis. In a follow-up of a previous report of liver injury caused by ampicillin, Köklü et al. [1] described a case of a 23-year-old man who redeveloped liver injury 17 days after starting a 10-day course of oral cefuroxime (bilirubin 0.7 mg/dl, alanine transaminase [ALT] 427 U/L, alkaline phosphatase [ALP] 646 U/L), which resolved within 2 months, suggesting cross-reactivity with ampicillin. In a report by Chalasani et al. for the Drug-Induced Liver Injury Network [2], among 300 cases of DILI in the United States collected between 2004 and 2008, 5 cases were attributed to cephalosporin, with single cases linked to cefuroxime. In another report, Ekiz et al. described a 60-year-old woman who developed jaundice 4 days after a 10-day course of oral cefuroxime (bilirubin 17.9 mg/dl rising to 30 mg/dl, ALT 1527 U/L, ALP 1006 U/L), with progressive worsening of international normalized ratio (INR) [1.9] and referral for transplant but with a subsequent full recovery [3]. Given the paucity of case reports of cefuroxime-induced DILI, this drug belongs to category D (one to four reported cases) according to a recently suggested categorization of drugs implicated in hepatotoxicity and DILI [4]. In this report, we present a case of a patient with paradoxical worsening of jaundice due to possible drug-induced cholestasis caused by cefuroxime following therapeutic endoscopic retrograde cholangiopancreatography (ERCP) for distal common bile duct (CBD) obstruction by a stone.
Case presentation
A previously healthy, 51-year-old Sri Lankan man presented with right upper quadrant colicky abdominal pain of 3 days’ duration. The pain was associated with yellow discoloration of the eyes, passage of dark urine, and generalized itching. He had no significant past medical, environmental, or social history. His clinical examination revealed that he was afebrile but had deep icterus, excoriations, and mild right upper quadrant abdominal tenderness. He had no stigmata of chronic liver disease.
The results of laboratory investigations included a normal full blood count and inflammatory markers, deranged liver biochemistry (total bilirubin 6.4 mg/dl, ALP 325 IU/L, aspartate transaminase [AST] 113 U/L, ALT 318 U/L), normal liver function (serum albumin 3.8 g/dl, serum globulin 2.6 g/dl, INR 1.00, activated partial thromboplastin time [APTT] 29 seconds), and normal renal profile. An ultrasound scan (USS) of the abdomen showed the presence of cholelithiasis with features of chronic cholecystitis and a dilated CBD and intrahepatic ducts due to a distal CBD obstruction. Contrast-enhanced computed tomography of the abdomen confirmed the presence of a distal CBD stone causing proximal CBD and intrahepatic duct dilation and cholelithiasis.
The patient underwent ERCP with sphincterotomy and balloon extraction of a CBD stone 2 weeks from the onset of symptoms. After surgery, the patient was given intravenous cefuroxime 750 mg three times daily for 1 day, followed by oral cefuroxime 500 mg twice daily for 5 days. The patient’s symptoms and biochemistry failed to improve, with worsening of cholestasis (total bilirubin 20.3 mg/dl, ALP 537 IU/L) following the “successful” therapeutic ERCP. Repeat ERCP 1 week later showed a normal CBD with no residual CBD stones. A 10-French, 10-cm CBD stent was inserted at this stage.
The patient was then referred to a hepatologist for evaluation of worsening jaundice post-ERCP. By this time, the patient’s obstructive jaundice symptoms were severe and disabling, and biochemical analysis revealed worsening cholestasis (total bilirubin 39 mg/dl, ALP 651 IU/L), relatively normal liver enzymes (AST 61 U/L, ALT 62 U/L, gamma-glutamyltransferase [GGT] 25 U/L] with normal liver function (serum albumin 3.7 g/dl, serum globulin 1.9 g/dl, INR 1.00, APTT 29 seconds), normal full blood count, normal inflammatory markers, and normal renal profile. Repeat USS of the abdomen revealed multiple cholelithiasis, chronic cholecystitis, stent in the CBD, and no intrahepatic biliary radical dilation. Test results for the patient’s hepatitis A immunoglobulin M (IgM), hepatitis E antibodies, hepatitis B surface antigen, anti-hepatitis C antibodies, and Leptospira IgM antibodies were negative.
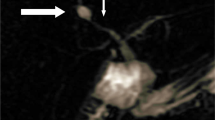
On the basis of all the above-mentioned information, an ultrasound-guided liver biopsy was performed. The patient was commenced on prednisolone 40 mg daily, ursodeoxycholic acid 300 mg three times daily, and cholestyramine 5 g three times daily for symptomatic relief. A liver biopsy showed no evidence of portal tract edema or inflammation, lesions or paucity of bile ducts, bile infarcts or leaks, or inflammation or interphase hepatitis. However, there was marked bilirubinostasis in zone 3 canaliculi, with surrounding hepatocytes revealing feathery degeneration and surrounding inflammation (Fig. 1). These features were compatible with intrahepatic cholestasis related to either drugs or sepsis.
One week later, the patient developed fever with chills and rigors, worsening cholestasis clinically and biochemically (total bilirubin 48 mg/dl, ALP 901 IU/L) with relatively normal liver enzymes (AST 55 U/L, ALT 91 U/L, GGT 43 U/L), raised inflammatory markers (C-reactive protein 36 mg/dl), and neutrophil leukocytosis (white blood cell count 14,400/mm3 with 80% neutrophils). A clinical diagnosis of cholangitis was made, and the patient was commenced on intravenous meropenem 1 g three times daily, after blood cultures, for 14 days. The CBD stent was removed endoscopically to exclude the possibility of a blocked stent as the precipitant of cholangitis. Oral prednisolone was tapered rapidly in view of the infection. The cholangitis episode settled with the antibiotics, with resolution of fever and normalizing of full blood count and inflammatory markers.
The patient’s cholestasis improved gradually over the next 2 months with ursodeoxycholic acid therapy. He had complete resolution of both clinical and biochemical features (total bilirubin 1.1 mg/dl, ALP 135 IU/L) of cholestasis 4 months into his illness. He was referred back to a hepatobiliary surgeon for elective laparoscopic cholecystectomy for residual cholelithiasis to prevent recurrence of complications and was advised to avoid cephalosporin use in the future. The timeline of the patient’s clinical course is presented in Table 1.
Discussion
This case is unique and complicated, involving a very rare DILI secondary to cefuroxime in the setting of therapeutic ERCP for distal CBD obstruction. Previously reported cases of DILI caused by cefuroxime were not complicated by preexisting obstructive jaundice.
The cholestasis in our patent, which was due to distal CBD obstruction by a stone, was expected to improve following therapeutic ERCP. However, despite successful stone extraction, CBD clearance, and stenting, our patient’s cholestasis paradoxically continued to worsen.
Worsening cholestasis post-ERCP may be due to a retained CBD stone, ascending cholangitis, a blocked or migrated stent, or drug-induced cholestasis. In our patient, the most likely reason was drug-induced cholestasis. This was suggested by the absence of features of sepsis following initial ERCP, the absence of ductal dilation seen on an USS with stent in situ in the CBD, and the features present on liver histology favoring intrahepatic cholestasis of drug-induced etiology. The agents that may be responsible for this may be the drugs used during or after ERCP. Our patient was given propofol, pethidine during ERCP, and cefuroxime post-ERCP. All of these agents are known to cause cholestasis, but cefuroxime seems the likely causative agent.
Cefuroxime is a second-generation cephalosporin very rarely known to cause DILI. Prior to the present report, fewer than five cases of cefuroxime causing DILI and cholestasis had been reported [1–3]. Although the exact mechanism of cholestatic DILI of cefuroxime is not known, it is likely idiosyncratic in nature and due to hypersensitivity. Conversely, a closely related third-generation cephalosporin, ceftriaxone, is known to cause biliary sludge formation because of the propensity of ceftriaxone to bind calcium and form insoluble crystals in bile in the gallbladder, resulting in biliary sludge or frank stones [5].
Our patient’s cholestasis worsened further following an episode of cholangitis, which was successfully treated with meropenem, thereby avoiding further exposure to cephalosporin. Following successful treatment of his cholangitis, the patient was managed with only ursodeoxycholic acid and cholestyramine. This resulted in gradual resolution of his cholestasis, with complete resolution in 4 months.
The place of steroids in drug-induced cholestasis is equivocal [6]. Corticosteroids can be effective for DILI associated with autoimmune or systemic hypersensitivity features. In our patient, there was no marked improvement of cholestasis on commencing steroids. Furthermore, the episode of cholangitis was worsened by steroids. Therefore, steroids were tapered rapidly at the onset of cholangitis with antibiotic cover.
Conclusions
Our patient’s case illustrates a rare DILI due to cefuroxime, presenting as prolonged cholestatic jaundice following “successful” therapeutic ERCP for an obstructing distal CBD stone. Clinicians must be aware of the causes of worsening cholestasis post-ERCP to manage as well as to prevent the recurrence of similar complications in such patients.
Abbreviations
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- APTT:
-
Activated partial thromboplastin time
- AST:
-
Aspartate transaminase
- CBD:
-
Common bile duct
- CRP:
-
C-reactive protein
- DILI:
-
Drug-induced liver injury
- ERCP:
-
Endoscopic retrograde cholangiopancreatography
- FBC:
-
Full blood count
- GGT:
-
Gamma-glutamyltransferase
- IgM:
-
Immunoglobulin M
- INR:
-
International normalized ratio
- USS:
-
Ultrasound scan
References
Köklü S, Yüksel O, Yolcu OF, Arhan M, Altiparmak E. Cholestatic attack due to ampicillin and cross-reactivity to cefuroxime. Ann Pharmacother. 2004;38:1539–40.
Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34.
Ekiz F, Usküdar O, Simsek Z, Yüksel I, Basar O, Altinbas A, et al. Cefuroxime axetil-induced liver failure. Ann Hepatol. 2010;9:306.
Björnsson ES, Hoofnagle JH. Categorization of drugs implicated in causing liver injury: critical assessment based upon published case reports. Hepatology. 2016;63:590–603.
Zinberg J, Chernaik R, Coman E, Rosenblatt R, Brandt LJ. Reversible symptomatic biliary obstruction associated with ceftriaxone pseudolithiasis. Am J Gastroenterol. 1991;86:1251–4.
Stine JG, Lewis JH. Current and future directions in the treatment and prevention of drug-induced liver injury: a systematic review. Expert Rev Gastroenterol Hepatol. 2016;10:517–36.
Acknowledgements
Not applicable.
Funding
Not applicable. No external source of funding was obtained in reporting this case.
Availability of data and materials
Data supporting this case report will be made available on request by the corresponding author.
Authors’ contributions
MAN drafted the initial manuscript. RSK, ASD, AP, JdSH, and HJdS completed a review of the literature and revised it critically for important intellectual content. All authors are accountable for all aspects of the work. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Niriella, M.A., Kumarasena, R.S., Dassanayake, A.S. et al. Worsening cholestasis and possible cefuroxime-induced liver injury following “successful” therapeutic endoscopic retrograde cholangiopancreatography for a distal common bile duct stone: a case report. J Med Case Reports 10, 371 (2016). https://doi.org/10.1186/s13256-016-1123-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-016-1123-0