Abstract
Introduction
There is an urgent unmet medical need for a safe, effective, nonopioid analgesic agent for postoperative pain control.
Methods
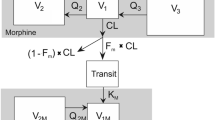
This first-in-man study was designed to explore a data-informed, model-based candidate dosage regimen and safety of a novel formulation of ketorolac tromethamine (NTM-001) delivered as a 12.5-mg intravenous (IV) bolus followed immediately by 3.5 mg/h continuous infusion over 24 h compared versus IV bolus dosing of 30 mg generic ketorolac every 6 h. The study evaluated pharmacokinetic parameters and safety profiles based on a targeted product profile. A graphical overlay method and model-based comparisons were used to assess the concentration–time curve.
Results
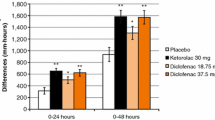
Healthy adults (n = 28, 50% men) received NTM-001 and bolus dosing in an open-label crossover design. Observed plasma concentrations were tightly aligned with predicted values with no outliers. Graphical overlay comparisons showed low between-subject variability and agreed with forecasted concentration–time targets. The pharmacokinetic (PK) base models fit with preliminary PK data from both the NTM-001 and bolus groups with model fit median profiles within 95% prediction limits and no updating of the models. Consistent with serum concentration–time profiles, pain relief scores fell within predicted limits, with initial pain relief scores of NTM-001 slightly above the target profile, likely because the initial serum ketorolac concentrations were somewhat higher than predicted. The 24-h pain relief predicted for NTM-001 based on the area under the median ketorolac pain relief versus time curve was about 6% below that of the pain relief target. Both treatments were well tolerated and no subject withdrew because of adverse events.
Conclusions
The PK parameters for NTM-001 and comparator bolus were similar to the modeling targets with no updating of the base model. There were no outliers and little intersubject variability. NTM-001 delivered as a bolus of 12.5 mg IV followed immediately by continuous infusion of 3.5 mg/h using a standard hospital infusion pump may offer an alternative to opioids for acute postoperative pain control.
Plain Language Summary
Opioids are effective analgesics but the risk for opioid use disorder (OUD) and opioid-associated side effects limit their use even for postoperative pain. Ketorolac is an established nonopioid pain reliever that may be as efficacious as morphine in this setting. This study evaluated a new ketorolac product (NTM-001) compared to generic ketorolac. Both were delivered using a standard hospital intravenous (IV) drug pump. The new ketorolac product was administered first with a loading dose of 12.5 mg followed immediately by a continuous IV infusion of 3.5 mg/h. This was compared to IV generic ketorolac administered as a bolus dose of 30 mg every 6 h. The study enrolled 28 healthy adult volunteers. As a crossover study, subjects underwent both treatments: once with the continuous infusion (NTM-001) and once with the IV injection every 6 h (bolus group) with a “washout” period in between. Blood was collected from the volunteers at several time-determined points during the 48-h study to chart ketorolac concentrations in the blood, which can be correlated to predicted levels of pain control. In this study, blood concentrations of ketorolac were reliably predictable and side effects were generally mild with no unexpected adverse events. The continuous infusion group achieved analgesic benefit at a lower total dose than did the every-6-h group over 24 h.






Similar content being viewed by others
Data Availability
The full trial protocol and datasets generated during or analyzed in the course of this study are available upon reasonable request from the corresponding author.
References
Sager ZS, Buss MK, Hill KP, Driver JA, Skarf LM. Managing opioid use disorder in the setting of a terminal disease: opportunities and challenges. J Palliat Med. 2020;23(2):296–9.
Mitra S, Carlyle D, Kodumudi G, Kodumudi V, Vadivelu N. New advances in acute postoperative pain management. Curr Pain Headache Rep. 2018;22(5):35.
Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315(15):1624–45.
Best MJ, McFarland EG, Anderson GF, Srikumaran U. The likely economic impact of fewer elective surgical procedures on US hospitals during the COVID-19 pandemic. Surgery. 2020;168(5):962–7.
Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–98.
De Luca ML, Ciccarello M, Martorana M, et al. Pain monitoring and management in a rehabilitation setting after total joint replacement. Medicine (Baltimore). 2018;97(40): e12484.
Mears SC, Edwards PK, Barnes CL. How to decrease length of hospital stay after total knee replacement. J Surg Orthop Adv. 2016;25(1):2–7.
Pergolizzi JV, Lequang JA, Passik S, Coluzzi F. Using opioid therapy for pain in clinically challenging situations: questions for clinicians. Minerva Anestesiol. 2019;85(8):899–908.
Mandema JW, Stanski DR. Population pharmacodynamic model for ketorolac analgesia. Clin Pharmacol Ther. 1996;60(6):619–35.
Toradol (ketorolac tromethamine tablets) Nutley, New Jersey: Roche Pharmaceuticals, 1997. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/019645s019lbl.pdf.
Ketorolac tromethamine injection USP Lake Forest, Illinois: Hospira; 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/074802s038lbl.pdf.
Motov S, Yasavolian M, Likourezos A, et al. Comparison of intravenous ketorolac at three single-dose regimens for treating acute pain in the emergency department: a randomized controlled trial. Ann Emerg Med. 2017;70(2):177–84.
Eidinejad L, Bahreini M, Ahmadi A, Yazdchi M, Thiruganasambandamoorthy V, Mirfazaelian H. Comparison of intravenous ketorolac at three doses for treating renal colic in the emergency department: a noninferiority randomized controlled trial. Acad Emerg Med. 2020;28:768–75.
Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23(6):415–27.
Brown CR, Mazzulla JP, Mok MS, Nussdorf RT, Rubin PD, Schwesinger WH. Comparison of repeat doses of intramuscular ketorolac tromethamine and morphine sulfate for analgesia after major surgery. Pharmacotherapy. 1990;10(6 Pt 2):45s–50s.
Gillis JC, Brogden RN. Ketorolac: a reappraisal of its pharmacodynamic and pharmacokinetic properties and therapeutic use in pain management. Drugs. 1997;53(1):139–88.
Sinha VR, Kumar RV, Singh G. Ketorolac tromethamine formulations: an overview. Expert Opin Drug Deliv. 2009;6(9):961–75.
Acknowledgements
The authors also wish to express their gratitude to all subjects who participated in this clinical trial.
Medical Writing/Editorial Assistance
Neumentum acknowledges the medical writing services of Jo Ann LeQuang of Angleton, Texas, USA, whose fees were covered by Neumentum.
Funding
This study was funded by Neumentum, Inc. Neumentum funded the study, the preparation of this article, and all fees associated with publication.
Author information
Authors and Affiliations
Contributions
All authors contributed to this paper and approved it for publication JVP and WS participated in the original study concept and design; WS contributed to statistical and data analysis. AB contributed to protocol and data analysis. All authors worked with the medical writer to draft the manuscript and all authors revised the manuscript independently. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Pergolizzi is the chief research and development officer at Neumentum, Inc. Ms. Batra is a clinical research associate at Neumentum, Inc. Dr. Schmidt is a consultant to Neumentum, Inc.
Ethical Approval
Ethical approval was provided by the IntegReview institutional review board (IRB) in Austin, Texas. The study complied with the Good Clinical Practice requirements described in the current International Council for Harmonization (ICH) guidelines and current federal regulations. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pergolizzi, J.V., Batra, A. & Schmidt, W.K. A Randomized Controlled Trial of a Novel Formulation of Ketorolac Tromethamine for Continuous Infusion (NTM-001) in Healthy Volunteers. Adv Ther 41, 659–671 (2024). https://doi.org/10.1007/s12325-023-02709-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02709-5