Abstract
Background
Diabetes mellitus (DM) plays an important role in restenosis and late in-stent thrombosis (ST). The current study using optical coherence tomography (OCT) aims to compare target lesion neointima in patients with or without diabetes after zotarolimus-eluting stent (ZES) treatment.
Methods
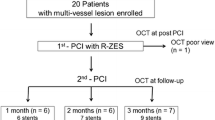
OCT images of 90,212 struts and quantitative coronary angiography (QCA) in 62 patients (32 with DM and 30 without DM) with 69 de novo coronary lesions (34 DM and 35 non-DM) both after ZES implantation and 12 ± 1 month angiographic follow-up were recorded. Patient characteristics, lesion characteristics, clinical outcomes, and OCT findings including neointimal thickness, coverage, malapposition, and intimal morphology were analyzed.
Results
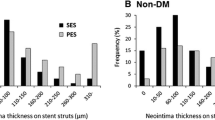
Baseline patient characteristics and lesion characteristics data were similar between the two groups. Higher neointimal thickness (0.14 ± 0.09 mm vs. 0.09 ± 0.04 mm, p = 0.021), more neovascularization (3.03 ± 6.24 vs. 0.52 ± 1.87, p = 0.017) and higher incidence of layered signal pattern (12.19 ± 19.91% vs. 4.28 ± 9.02%, p = 0.049) were observed in diabetic lesions comparing with non-diabetic lesions. No differences were found in malapposition, uncovered percentage, and thrombus between the two groups (all p > 0.05). Occurrence of clinical adverse events was also similar during the follow-up period (p > 0.05).
Conclusion
Although more neointimal proliferation and more neovascularization were found in diabetic coronary lesions when compared with non-diabetic lesions, treatment with ZES showed similar stent malapposition rate at 1-year follow-up. The data indicated that ZES treatment could possibly be effective in treating diabetic coronary lesions.
Trial Registration
ClinicalTrials.gov identifier, NCT01747356.

Similar content being viewed by others
References
Navarese EP, Tandjung K, Claessen B, et al. Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: comprehensive network meta-analysis. BMJ. 2013;347:f6530.
Sabate M, Cequier A, Iniguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482–90.
Stone GW, Ellis SG, Cannon L, et al. Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294:1215–23.
Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2015;65:2496–507.
Kastrati A, Massberg S, Ndrepepa G. Is diabetes the achilles’ heel of limus-eluting stents? Circulation. 2011;124:869–72.
Hong SJ, Kim MH, Ahn TH, et al. Multiple predictors of coronary restenosis after drug-eluting stent implantation in patients with diabetes. Heart. 2006;92:1119–24.
Ndrepepa G, King L, Kastrati A. More deserved focus on diabetic patients. Circ Cardiovasc Intervent. 2011;4:115–7.
Du R, Zhang RY, Lu L, et al. Increased glycated albumin and decreased esRAGE levels in serum are related to negative coronary artery remodeling in patients with type 2 diabetes: an intravascular ultrasound study. Cardiovasc Diabetol. 2018;17:149.
Abali G, Akpinar O, Soylemez N. Correlation of the coronary severity scores and mean platelet volume in diabetes mellitus. Adv Ther. 2014;31:140–8.
Lin BY, Li P, Wu P, Jiang RN, Bundhun PK, Ahmed MA. Duration of dual antiplatelet therapy and late stent thrombosis following percutaneous coronary intervention with second-generation drug-eluting stents: a simple meta-analysis of randomized controlled trials. Adv Ther. 2019;36:3166–73.
Yeung AC, Leon MB, Jain A, et al. Clinical evaluation of the resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J Am Coll Cardiol. 2011;57:1778–83.
Lowik MM, Lam MK, Sen H, et al. Safety of second-generation drug-eluting stents 3 years after randomised use in the TWENTE trial. EuroIntervention. 2015;10:1276–9.
Silber S, Serruys PW, Leon MB, et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global RESOLUTE program. JACC Cardiovasc Intervent. 2013;6:357–68.
Saito S, Maehara A, Vlachojannis GJ, Parise H, Mehran R, RESOLUTE Japan Investigators. Clinical and angiographic evaluation of the resolute zotarolimus-eluting coronary stent in Japanese patients—long-term outcome in the RESOLUTE Japan and RESOLUTE Japan small vessel study. Circ J. 2015;79:96–103.
Meredith IT, Worthley S, Whitbourn R, et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc Intervent. 2009;2:977–85.
Neumann FJ, Widimsky P, Belardi JA. One-year outcomes of patients with the zotarolimus-eluting coronary stent: RESOLUTE international registry. EuroIntervention. 2012;7:1181–8.
Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010;363:136–46.
Qiao S, Chen L, Chen S, Wang W, Zhu G. One-year outcomes from an all-comers Chinese population of patients implanted with the resolute zotarolimus-eluting stent. Am J Cardiol. 2014;113:613–20.
Zhu Z, Wu Y, Shen Z, et al. Safety and efficacy of zotarolimus-eluting stents in the treatment of diabetic coronary lesions in Chinese patients: the RESOLUTE-DIABETES CHINA Study. J Diabetes. 2019;11:204–13.
Nammas W, Ligthart JM, Karanasos A, Witberg KT, Regar E. Optical coherence tomography for evaluation of coronary stents in vivo. Expert Rev Cardiovasc Ther. 2013;11:577–88.
Alfonso F, Sandoval J, Cardenas A, Medina M, Cuevas C, Gonzalo N. Optical coherence tomography: from research to clinical application. Minerva Med. 2012;103:441–64.
Lee KS, Lee JZ, Hsu CH, et al. Temporal trends in strut-level optical coherence tomography evaluation of coronary stent coverage: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2016;88:1083–93.
Kuroda M, Shinke T, Sakaguchi K, et al. Association between daily glucose fluctuation and coronary plaque properties in patients receiving adequate lipid-lowering therapy assessed by continuous glucose monitoring and optical coherence tomography. Cardiovasc Diabetol. 2015;14:78.
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Geneva: WHO Department of Non communicable Disease Surveillance; 1999.
Iwasaki M, Otake H, Shinke T, et al. Vascular responses in patients with and without diabetes mellitus after everolimus-eluting stent implantation. Circ J. 2014;78:2188–96.
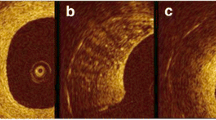
Ong DS, Jang IK. Fundamentals of optical coherence tomography: image acquisition and interpretation. Interv Cardiol Clin. 2015;4:225–37.
Gonzalo N, Serruys PW, Okamura T, et al. Optical coherence tomography patterns of stent restenosis. Am Heart J. 2009;158:284–93.
Tearney GJ, Regar E, Akasaka T, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–72.
Kim JS, Kim BK, Jang IK, et al. ComparisOn of neointimal coVerage betwEen zotaRolimus-eluting stent and everolimus-eluting stent using Optical Coherence Tomography (COVER OCT). Am Heart J. 2012;163:601–7.
Tanaka N, Terashima M, Rathore S, et al. Different patterns of vascular response between patients with or without diabetes mellitus after drug-eluting stent implantation: optical coherence tomographic analysis. JACC Cardiovasc Interv. 2010;3:1074–9.
Kume T, Akasaka T, Kawamoto T, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006;97:1713–7.
Gao L, Park SJ, Jang Y, et al. Comparison of neoatherosclerosis and neovascularization between patients with and without diabetes: an optical coherence tomography study. JACC Cardiovasc Interv. 2015;8:1044–52.
Gutierrez-Chico JL, van Geuns RJ, Regar E, et al. Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs. a fluoropolymer-coated everolimus-eluting stent at 13-month follow-up: an optical coherence tomography substudy from the RESOLUTE All Comers trial. Eur Heart J. 2011;32:2454–63.
Claessen BE, Henriques JPS, Vendrik J, et al. Paclitaxel-eluting balloon versus everolimus-eluting stent in patients with diabetes mellitus and in-stent restenosis: insights from the randomized DARE trial. Catheter Cardiovasc Interv. 2019;93:216–21.
Tian F, Chen Y, Liu H, Zhang T, Guo J, Jin Q. Assessment of characteristics of neointimal hyperplasia after drug-eluting stent implantation in patients with diabetes mellitus: an optical coherence tomography analysis. Cardiology. 2014;128:34–40.
Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–50.
Suzuki N, Kozuma K, Kyono H, et al. Predominant microvessel proliferation in coronary stent restenotic tissue in patients with diabetes: insights from optical coherence tomography image analysis. Int J Cardiol. 2013;168:843–7.
Armstrong EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes mellitus. Circulation. 2013;128:1675–85.
Du R, Zhang RY, Zhu ZB, et al. Impact of angiographic and intravascular ultrasound features on clinical outcome after sirolimus-eluting stent implantation for de-novo lesions in nondiabetic and type 2 diabetic patients. Coron Artery Dis. 2010;21:175–81.
Zhu ZB, Zhang RY, Zhang Q, et al. Moderate–severe renal insufficiency is a risk factor for sirolimus-eluting stent thrombosis. The RIFT study. Cardiology. 2009;112:191–9.
Tahara S, Chamie D, Baibars M, Alraies C, Costa M. Optical coherence tomography endpoints in stent clinical investigations: strut coverage. Int J Cardiovasc Imaging. 2011;27:271–87.
Zhu J, Yang K, Jing Y, et al. The effects of low-dose nepsilon-(carboxymethyl)lysine (CML) and nepsilon-(carboxyethyl)lysine (CEL), two main glycation free adducts considered as potential uremic toxins, on endothelial progenitor cell function. Cardiovasc Diabetol. 2012;11:90.
Maeng M, Tilsted HH, Jensen LO, et al. Differential clinical outcomes after 1 year versus 5 years in a randomised comparison of zotarolimus-eluting and sirolimus-eluting coronary stents (the SORT OUT III study): a multicentre, open-label, randomised superiority trial. Lancet. 2014;383:2047–56.
Xu B, Yang Y, Yuan Z, et al. Zotarolimus- and paclitaxel-eluting stents in an all-comer population in China: the RESOLUTE China randomized controlled trial. JACC Cardiovasc Interv. 2013;6:664–70.
Belardi JA, Widimsky P, Neumann FJ, Mauri L, Albertal M, Investigators RI. Real-world safety and effectiveness outcomes of a zotarolimus-eluting stent: final 3-year report of the RESOLUTE International study. J Interv Cardiol. 2013;26:515–23.
Acknowledgements
The authors would like to thank Mr. Yu Linjun and Miss. Liu Lili for their help with PCI and OCT image blinding and data collection.
Funding
The study and rapid service fee was sponsored by Science and Technology Commission of Shanghai Municipality (ID 16DZ1930402), Shanghai Municipal Health and Family Planning Commission (ID 2013ZYJB1002), and Shanghai Jiao Tong University (ID YG2013MS31).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Dr. Zhengbin Zhu, Dr. Jinwei Ni, Dr. Fenghua Ding, and Dr. Zhenkun Yang performed the angiography procedure and OCT study. Dr. Zhengbin Zhu and Dr. Haotian Zhang analyzed all clinical data and wrote the manuscript. Dr. Jinzhou Zhu and Dr. Weiwei Quan were responsible for patient follow-up and record all data. Dr. Run Du analyzed OCT images. Dr. Jian Hu and Prof. Ruiyan Zhang designed this study and observed the whole study process. Zhengbin Zhu, Jinzhou Zhu, and Run Du contributed equally.
Disclosures
Zhengbin Zhu, Jinzhou Zhu, Run Du, Haotian Zhang, Jinwei Ni, Weiwei Quan, Jian Hu, Fenghua Ding, Zhenkun Yang, and Ruiyan Zhang have nothing to disclose.
Compliance with Ethics Guidelines
The study protocol was approved by the Ethic Committee of Ruijin hospital, and each patient signed informed consent. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Data Availability
All data and imaging material of this study is available. The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11871414.
Rights and permissions
About this article
Cite this article
Zhu, Z., Zhu, J., Du, R. et al. Efficacy of Zotarolimus-Eluting Stents in Treating Diabetic Coronary Lesions: An Optical Coherence Tomography Study. Adv Ther 37, 1579–1590 (2020). https://doi.org/10.1007/s12325-020-01273-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01273-6