Abstract
Introduction
Food can alter the pharmacokinetics of certain abuse-deterrent formulations. Morphine ARER is an oral abuse-deterrent formulation of ER morphine sulfate tablets formulated with physical and chemical properties that contribute to the abuse-deterrent aspects of the drug. This study compared the relative bioavailability of Morphine ARER in the presence and absence of food.
Methods
This was a randomized, single-dose, two-treatment, crossover study in which healthy adults received Morphine ARER 100 mg under fasting and fed conditions. Subjects were given naltrexone 50 mg to limit opioid effects. Plasma concentrations of morphine and its active metabolite morphine-6-glucuronide (M6G) were obtained up to 48 h post-dose; area under the plasma concentration-time curve (AUC) from time 0 extrapolated to infinity (AUC0–∞), maximum observed plasma concentration (Cmax) and time to Cmax (Tmax) were calculated. Safety was evaluated by observation or report of adverse events, which were monitored during the treatment periods.
Results
Of 28 enrolled subjects, 27 completed all treatments; 1 subject in the fasted group withdrew voluntarily. Under fed conditions, the Cmax for morphine was 33% higher (44.78 vs. 33.30 ng/ml for fed and fasted conditions, respectively) and the median Tmax was 30 min longer than under fasted conditions. The overall morphine exposure (AUC0–∞) was similar for fed (440.6 ng · h/ml) vs. fasted conditions (395.1 ng · h/ml). For M6G, the Cmax and AUC0–∞ were similar under both conditions, and the median Tmax for M6G was 60 min longer under fed conditions. Common adverse events were somnolence and nausea.
Conclusion
Morphine ARER can be administered without regard to food.
Plain Language Summary
Plain language summary available for this article.
Funding
Inspirion Delivery Sciences, LLC.
Plain Language Summary
Food alters how the body processes some currently available opioids. How the opioid is formulated in the final commercial product can impact this effect. Morphine ARER is a new oral abuse-deterrent formulation of extended-release morphine created with properties to make it more difficult to abuse via the intranasal and intravenous routes. To better understand how food affects Morphine ARER bioavailability, we compared the amount of morphine in the blood when 100 mg of Morphine ARER was given with or without food, in random order, to 27 healthy volunteers. Plasma samples were collected up to 48 h after dosing to measure the concentrations of morphine and its active metabolite morphine-6-glucuronide. We measured the amount of drug absorbed by using the area under the plasma concentration-time curve (AUC) and the rate of drug absorption by looking at the highest amount of drug observed in the blood using the maximum observed plasma concentration (Cmax) and time to Cmax (Tmax). When subjects were fed, the Cmax for morphine was 33% higher (44.78 ng/ml) than when they fasted (33.30 ng/ml). The median Tmax was 30 min longer when subjects were fed. Total morphine exposure (AUC0–∞) was similar when subjects were fed (440.6 ng · h/ml) or when they fasted (395.1 ng · h/ml). Safety was evaluated throughout the treatment periods by adverse events, either observed by the clinician or reported by subjects. The most common adverse events noted were somnolence (e.g., sleepiness) and nausea. Our findings show that Morphine ARER has similar bioavailability when taken with or without food.
Similar content being viewed by others
Introduction
Misuse and abuse of opioids continue to be a public health issue, with preliminary data from 2017 estimating > 47,000 deaths in the USA caused by opioids [1]. Extended-release (ER) opioid formulations are often misused and abused because they contain high amounts of opioid [2]. Abusers often try to manipulate ER formulations in an attempt to convert the ER properties into immediate release, allowing for more rapid euphoric effects. [2] Additionally, opioid formulations that are more easily prepared for non-oral routes of administration [e.g., intravenous (IV), intranasal] are more attractive to abusers as those routes can also lead to a more rapid euphoric effect [3]. Abuse-deterrent formulations (ADFs) of opioids are one approach to combat prescription opioid misuse and abuse [4]. Opioid ADFs provide pain relief while deterring common methods of manipulation and extraction to reduce the potential for abuse and misuse [4].
Morphine ARER (MorphaBond™ ER, Daiichi Sankyo, Inc., Basking Ridge, NJ, USA) is a novel oral ADF of ER morphine sulfate tablets. [5]. Morphine ARER is formulated with proprietary SentryBond™ technology, which has physical and chemical properties that contribute to the abuse-deterrent aspects of the drug [5]. The active ingredient is contained within a polymer matrix of inactive ingredients and is difficult to visually distinguish or physically separate from the polymer matrix (data on file). Morphine ARER is expected to deter abuse by the intranasal and IV routes of administration; however, abuse by intranasal, IV and oral routes is still possible.
Food can alter the pharmacokinetics (PK) of certain abuse-deterrent formulations [6]. High-fat meals may increase total exposure to the drug and delay the maximum plasma concentration or increase the oral bioavailability; these PK changes can alter the effectiveness of the opioid, which could result in poorly managed pain [6]. The goal of this study was to compare the relative bioavailability of Morphine ARER under fed and fasted conditions.
Methods
Subjects
Healthy male and female subjects aged 18–45 years with a body mass index (BMI) between 18.0 and 30.0 kg/m2 were enrolled in the study. Health assessments at the screening were used to determine good health and lack of clinically significant abnormalities. Female subjects were required to abstain from sexual intercourse or use a reliable method of contraception. Females who were pregnant, lactating or had a high likelihood of pregnancy during the study were excluded. Other exclusions were opioid-naive subjects, those with a history of considerable respiratory disease or concurrent infectious disease, system disorder or organ dysfunction, or gastrointestinal disorders.
Study Design and Treatments
This was a randomized, single-dose, two-period, two-treatment, two-sequence crossover study conducted under fasted and fed conditions. Treatments were administered according to a two-treatment, two-sequence randomization schedule (AB or BA). The randomization was generated by the Biostatistics Department of Novum Pharmaceutical Research Services before the first dosing period using SAS version 9.2 software. In treatment period 1, subjects fasted overnight for at least 10 h before receiving a single-dose Morphine ARER 100-mg tablet. In treatment period 2, subjects fasted overnight for at least 10 h and were then given a standard high-fat, high-calorie breakfast before receiving a single-dose Morphine ARER 100-mg tablet. A 7-day interval occurred between the doses. A 50 mg oral naltrexone tablet with 240 ml of water was provided to subjects at 12 h ± 30 min and 1.5 h ± 30 min before dosing with morphine and 12 h ± 30 min after dosing with morphine to minimize opioid side effects.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Novum Independent Institutional Review Board (NIIRB) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Pharmacokinetic Assessments
Blood samples from subjects were collected pre-dose (time 0) and post-dose at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 6, 8, 10, 12, 16, 24, 30, 36 and 48 h. At each time point, 10 ml of blood was collected in K3 EDTA vacutainers. Parameters of the following were used to evaluate the plasma concentration of morphine and active metabolite morphine-6-glucronide (M6G): maximum observed plasma concentration (Cmax), time to maximum observed plasma concentration (Tmax), and area under the plasma concentration-time curve from time 0 to the last measurable concentration (AUC0–t), extrapolated to infinity (AUC0–∞), and at various post-dose time points. Plasma concentrations of morphine and M6G were measured using a validated analytical procedure completed by Sannova Analytical, Inc. (Somerset, NJ, USA).
Safety Assessment
The Medical Dictionary for Regulatory Activities (MedDRA version 15.0) was used to code adverse events (AEs). AEs were reported and observed throughout the treatment periods to assess safety. Severity of the AEs was determined by the clinic staff, whereas the significance of the AEs to the study treatment was determined by the investigator.
Statistical Analysis
A sample size of approximately 28 dosed subjects was deemed sufficient to meet the objectives of the study. All pharmacostatistical calculations were completed using SAS version 9.2 or later (Cary, NC, USA). Analysis of variance was completed using the general linear model procedure of the statistical software. The least squares mean for the treatments was calculated, and 90% confidence intervals (CIs) were made based on geometric mean ratios taken from logarithmic transformed data.
Results
Subjects
Of the 28 subjects who were enrolled, 27 completed the study. One subject voluntarily withdrew from the study because of personal reasons before period 2 check-in; that subject received Morphine ARER under fasted conditions in period 1. Subject demographics are shown in Table 1.
Pharmacokinetics
Morphine
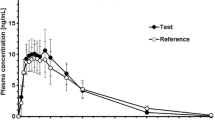
Under fed conditions, the Cmax for morphine was 33% higher (44.78 vs. 33.39 ng/ml for fed and fasted conditions, respectively), and the median Tmax was 30 min longer (4.5 h vs. 4.0 h, respectively; Fig. 1). The overall morphine exposures (AUC0–∞) were similar (440.6 ng · h/ml vs. 395.1 ng · h/ml, respectively). The 90% CI for Cmax was outside the 80–125% equivalence range for fed vs. fasted states (Table 2). There was no change in the overall extent of bioavailability, with the 90% CI for both morphine AUC0–t and AUC0–∞ geometric mean ratios (fed vs. fasted) falling within the 80–125% range.
M6G
Under fed vs. fasted conditions, the Cmax for M6G was similar (fed, 193.2 ng/ml; fasted, 196.8 ng/ml) and the median Tmax was 60 min longer (fed, 4.5 h; fasted, 3.5 h; Fig. 2). The overall M6G exposure (AUC0–∞) was similar for each state (fed, 2035.4 ng · h/ml; fasted, 2058.7 ng · h/ml). The 90% CIs for geometric mean ratios of Cmax, AUC0–∞ and AUC0–t were all within the equivalence range for M6G (Table 3).
Safety
A total of 11 AEs were reported by 10 of the 28 subjects who participated in the study (Table 4). All AEs were considered mild and resolved spontaneously before study completion. The most common AEs were somnolence and nausea.
Discussion
Food can alter the PK of certain ADFs, so it is important to consider the effect of food on the PK of new opioid formulations as they become available [6]. There are two recent examples in which the importance of food intake on an ADF was highlighted. The first example is that of Avridi™ (oxycodone hydrochloride immediate-release tablets, Purdue Pharma LP, Stamford, CT, USA), for which the US Food and Drug Administration did not recommend the approval because of the significant delay in oxycodone release in the presence of food [7]. The product required dosing on an empty stomach, or pain control might have been inadequate [7]. The second example is Xtampza® ER (oxycodone ER, Collegium Pharmaceutical, Canton, MA, USA) [8, 9]. The oral bioavailability of oxycodone from this formulation is greater when taken with food, and patients are therefore instructed to take their doses with approximately the same amount of food each day to ensure consistent plasma levels are achieved and to minimize fluctuations in analgesia [8, 9].
In this single-dose bioavailability study, morphine Cmax was 33% higher and Tmax was 30 min longer under fed conditions compared with fasted conditions. Overall morphine exposure (AUC0–∞) was 12% greater in the fed state; however, this difference was within the 80–125% equivalence range. The morphine metabolite, M6G, exhibited a similar Cmax and total exposure, but had a 60-min longer Tmax in the fed vs. fasted state. Although morphine Cmax was slightly outside the bioequivalence limit, we and the FDA did not consider these differences to be clinically relevant given that the overall exposure was similar for both morphine and M6G. These data suggest that Morphine ARER can be administered without regard to food intake and require no specific food-related dosing requirements in the prescribing information. In comparison, a currently marketed oxycodone ADF exhibits a 50–60% increase in AUC when administered with food, requiring a recommendation in the prescribing information to instruct patients to take each dose of the formulation with the same amount of food to avoid plasma drug level fluctuations [9]. Despite slight increases in Cmax, Morphine ARER maintains its ER profile and would likely not be utilized by a potential abuser who prefers rapid absorption to induce euphoria. Morphine ARER is expected to deter abuse by the intranasal and IV routes of administration; however, abuse by the intranasal, oral and IV routes is still possible.
A limitation of this study is the potential effects of using naltrexone to minimize the side effects of morphine. Naltrexone exhibits a similar AE profile to that of opioids (primarily nausea) and may affect gastric transit [10]. However, the FDA recommends the use of naltrexone in PK studies of opioid ADFs. Another limitation is the single-dose study design, which does not allow for assessment of steady-state drug levels and of a more realistic variable diet. However, because subjects received a high-fat diet, it is likely that the 12% increase in AUC is the maximum possible exposure, and any varied diet would result in a lower exposure to morphine.
Conclusion
In this single-dose PK study, Morphine ARER had similar bioavailability under fed and fasted conditions, indicating that Morphine ARER can be administered without regard to food intake.
References
Centers for Disease Control and Prevention. National vital statistics reports rapid release: provisional drug overdose death counts for 2017. 2018. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. Accessed 24 Aug 2018.
Moorman-Li R, Motycka CA, Inge LD, et al. A review of abuse-deterrent opioids for chronic nonmalignant pain. P T. 2012;37:412–8.
Katz N, Dart RC, Bailey E, et al. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37:205–17.
Pergolizzi JV Jr, Raffa RB, Taylor R Jr, et al. Abuse-deterrent opioids: an update on current approaches and considerations. Curr Med Res Opin. 2018;34:711–23.
MorphaBond ER (morphine sulfate) extended-release tablets [prescribing information]. Basking Ridge, NJ: Daiichi Sankyo, Inc.; September 2018. 2018. https://morphabondhcp.com/prescribing-information-portlet/getDocument?product=MB&inline=true. Accessed 24 Oct 2018.
Singh BN. Effects of food on clinical pharmacokinetics. Clin Pharmacokinet. 1999;37:213–55.
FDA briefing document (Avridi), Joint Meeting of Anesthetic and Analgesic Drug Products Advisory Committee and Drug Safety and Risk Management Advisory Committee, September 10, 2015. https://www.fdanews.com/ext/resources/files/09-15/9-15-FDA-Briefing.pdf?1520642200. Accessed 24 Oct 2018.
FDA briefing document (Xtampza ER), Joint Meeting of Anesthetic and Analgesic Drug Products Advisory Committee and Drug Safety and Risk Management Advisory Committee, September 11, 2015. https://www.pharmamedtechbi.com/~/media/Supporting%20Documents/The%20Pink%20Sheet%20DAILY/2015/September/Collegium_oxycodone_AC_company_brfg.pdf. Accessed 24 Oct 2018.
Xtampza ER (oxycodone) extended-release capsules [prescribing information]. Stoughton, MA: Collegium Pharmaceuticals, Inc; September 2018. 2018 http://www.xtampzaer.com/assets/pdf/xtampza-pi.pdf.
Naltrexone hydrochloride tablets USP [prescribing information]. Webster Groves, MO: Mallinckrodt Pharmaceuticals; July 2017. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/018932s017lbl.pdf. Accessed 24 Oct 2018.
Acknowledgements
The authors and study investigators thank the participants of this study.
Funding
This study was funded by Inspirion Delivery Sciences, LLC. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. Daiichi Sankyo, Inc., funded the journal’s article processing charges and open access fee.
Medical Writing Assistance
Medical writing support (funded by Daiichi Sankyo, Inc.) was provided by Kelly M. Cameron, PhD, ISMPP Certified Medical Publication Professional, of JB Ashtin, who, on the behalf of the authors, developed the first draft based on an author-approved outline and assisted in implementing author revisions throughout the editorial process.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
The information in this manuscript was previously presented at the International Conference on Opioids in Boston, MA, on June 10–12, 2018, and PAIN Week in Las Vegas, NV, on September 4–8, 2018. Please contact the corresponding author for additional information.
Disclosures
Eric R. Kinzler is a full-time employee of and/or consultant for Inspirion Delivery Sciences. Carmela Pantaleon is a full-time employee of and/or consultant for Inspirion Delivery Sciences. Matthew S. Iverson is a full-time employee of and/or consultant for Inspirion Delivery Sciences. Stefan Aigner is a full-time employee of and/or consultant for Inspirion Delivery Sciences.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Novum Independent Institutional Review Board (NIIRB) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Data Availability
Please contact the corresponding author for data requests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8299388.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kinzler, E.R., Pantaleon, C., Iverson, M. et al. A Randomized, Crossover Study on the Effect of Food on the Pharmacokinetic Characteristics of Morphine ARER (MorphaBond™ ER), an Abuse-Deterrent Formulation of Extended-Release Morphine. Adv Ther 36, 2394–2401 (2019). https://doi.org/10.1007/s12325-019-01022-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-01022-4