Abstract
Introduction
Immune-mediated inflammatory diseases (IMIDs) are chronic autoimmune conditions that share common pathophysiologic mechanisms. The optimal management of patients with IMIDs remains challenging because the coexistence of different conditions requires the intervention of several specialists. The aim of this study was to develop a series of statements defining overarching principles that guide the implementation of a multidisciplinary approach for the management of spondyloarthritis (SpA)-related IMIDs including SpA, psoriasis, psoriatic arthritis, Crohn’s disease, ulcerative colitis and uveitis.
Methods
A Delphi consensus-based approach was used to identify a core set of statements. The process included development of initial questions by a steering committee, an exhaustive search of the literature using complementary approaches to identify potential statements and two Delphi voting rounds for finalization of the statements.
Results
Consensus was achieved on the related nature of IMIDs, the existence of a high prevalence of multiple IMIDs in a single patient and the fact that a multidisciplinary approach can result in a more extensive evaluation and comprehensive approach to treatment. The goals of a multidisciplinary team should be to increase diagnosis of concomitant IMIDs, improve the decision-making process, and increase patient satisfaction and adherence. Early referral and diagnosis, early recognition of concomitant IMIDs and optimizing treatment to improve patient quality of life are some of the advantages of using multidisciplinary teams. To be effective, a multidisciplinary team should be equipped with the appropriate tools for diagnosis and follow-up, and at a minimum the multidisciplinary team should include a dermatologist, gastroenterologist and rheumatologist; providing psychologic support via a psychologist and involving an ophthalmologist, general practitioners and nurses in multidisciplinary care is also important.
Conclusion
The present Delphi consensus identified a set of overarching principles that may be useful for implementation of a multidisciplinary approach for the management of SpA-related IMIDs.
Funding
Aristea and Hippocrates.
Similar content being viewed by others
Introduction
The term “immune-mediated inflammatory diseases” (IMIDs) defines a group of chronic autoimmune conditions that share common pathophysiologic mechanisms [1, 2]. A large body of evidence shows that multiple IMIDs can coexist within the same patient and within families, as a consequence of shared genetic predisposing factors [1, 3]. The fact that distinct IMIDs, including psoriasis (Pso), psoriatic arthritis (PsA), spondyloarthritis (SpA) and inflammatory bowel disease (IBD), may be effectively treated by targeting the same inflammatory mediator [e.g., tumor necrosis factor-α (TNF-α)] further demonstrates a common underlying pathophysiology [2, 4].
With the introduction of biologic therapies over the past 2 decades, remarkable progress has been achieved in the treatment of individual IMIDs. However, optimal management of patients with IMIDs remains challenging because the coexistence of different conditions requires the intervention of several specialists, typically a rheumatologist, dermatologist and gastroenterologist. Validated strategies for an integrated and comprehensive assessment and treatment of IMIDs that consider all disease manifestations, instead of considering each IMID individually, are lacking or are just beginning to emerge in various settings, including SpA, IBD and psoriatic disease [5,6,7,8]. The design of such strategies is complex and requires a close collaboration between specialists.
As far as the management of IMID patients is concerned, multidisciplinary care provided by a team composed of several healthcare professionals is considered a valuable approach and is widely recommended. For example, the guidelines for the management and treatment of rheumatoid arthritis (RA) patients issued in 2009 by the UK National Institute for Health and Clinical Excellence (NICE) emphasize the importance of comprehensive management of patients with RA [9]. Recommendations from many scientific societies for the main SpA-related IMIDs, including PsA and axial SpA, stress the importance of the multidisciplinary approach in the management of these diseases. Overarching principle 2 from the 2015 GRAPPA treatment recommendations for patients with PsA states that “Multidisciplinary and multispecialty assessment and management will be most beneficial for individual patients” [10], the 2015 update of the EULAR recommendations for the management of PsA overarching principle C states that “Rheumatologists are the specialists who should care for the musculoskeletal manifestations of patients with PsA; in the presence of clinically significant skin involvement a rheumatologist and a dermatologist should collaborate in diagnosis and management” [6], and the 2016 update of the ASAS-EULAR management recommendations for axial SpA overarching principle 1 states that “Axial SpA is a potentially severe disease with different manifestations, usually requiring multidisciplinary management coordinated by the rheumatologist” [11].
However, the evidence supporting the effectiveness of this strategy is very limited. Most clinical studies comparing management by a multidisciplinary team with conventional management are from the rheumatology field and their results are controversial [12, 13]. Moreover, establishing a multidisciplinary team is a demanding process that may not be feasible [14,15,16]. The composition of the multidisciplinary team and other characteristics may vary according to different settings. However, regardless of the setting, formal coordination of the team and common definition of the goals are crucial for the successful implementation of this approach [14].
To encourage the adoption of a multidisciplinary approach for the management of SpA-related IMIDs including SpA, Pso, PsA, IBD [Crohn’s disease (CD) and ulcerative colitis (UC)] and uveitis, a panel of experts in rheumatology, dermatology, gastroenterology and ophthalmology set out to define overarching principles that guide the implementation of such an approach. Toward this aim, questions addressing the relevant issues regarding multidisciplinary care for SpA-related IMIDs were drafted by a steering committee, and a systematic review of the literature was performed to answer these questions, producing preliminary statements. Two rounds of Delphi were then conducted among the panel of experts to identify the final 13 consensus statements presented in this article, each referring to one of the discussed questions. The main objective of these statements is to inform dermatologists, gastroenterologists and rheumatologists about the rationale and value of an integrated approach to SpA-related IMIDs and to promote and guide towards the institution of multidisciplinary teams for the management of patients affected by these conditions.
Methods
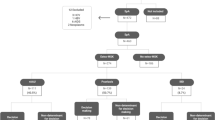
The consensus statements presented in this article were produced within the BRIDGE (Be Refocused on Immunology, Dermatology, Gastroenterology and Rheumatology) project, aimed at promoting collaboration among IMID specialists. The main objective of the process was to produce a series of statements defining overarching principles of the multidisciplinary management of SpA-related IMIDs including SpA, Pso, PsA, CD, UC and uveitis, supported by published evidence or based on expert consensus. Consensus was generated by means of a modified Delphi method, an interactive technique that develops consensus in two or more rounds of questions submitted to a panel of experts [17,18,19]. The consensus-finding process (Fig. 1) consisted of three phases (phases 1, 2 and 3) and took from April 2016 to March 2017 to complete. This article does not contain any novel studies with human or animal subjects performed by any of the authors.
Phase 1
In April 2016, a steering committee composed of a dermatologist (GG), gastroenterologist (FR) and rheumatologist (IO) met to identify IMID experts who could be part of the scientific board to define the objectives and topics covered by the statements and to plan the entire consensus-finding process. The IMID experts, seven rheumatologists, seven dermatologists, six gastroenterologists and one ophthalmologist, were selected based on their publication record in the IMID field and previous contributions to similar activities; most of these experts had direct experience (i.e., clinical practice) in the multidisciplinary management of SpA-related IMIDs. The steering committee drafted nine questions addressing relevant issues associated with the multidisciplinary approach to SpA-related IMIDs and proposed answers based on published evidence and expert opinion. The questions addressed the following issues: (1) the context and rationale for adopting a multidisciplinary approach; (2) the value of the multidisciplinary approach throughout all stages of patient management; (3) goals of the multidisciplinary team; (4) composition of the multidisciplinary team. To provide further evidence-based support to the answers, a systematic review of the literature was planned. In a second meeting held in July 2016 and attended by the entire scientific board (steering committee and experts), the nine questions and their answers were finalized and the details of the systematic search of the literature were established.
Phase 2
A systematic PubMed search was performed using pre-defined key words and inclusion criteria. Based on the evidence extracted from the selected articles, the answers to the nine questions were defined and produced a total of 52 statements. At a third meeting held in October 2016, the preliminary version of the statements was presented to the scientific board; the statements were then reviewed and finalized to produce the first version of the statements to be submitted to the first round of the Delphi process. Great care was taken to reduce redundancy and to improve the clarity and readability of the statements. The questions were reduced from 9 to 7 and the number of related statements was reduced from 52 to 13 by removing repetitions and, when possible, by collapsing multiple statements.
The first Delphi round was performed online: the document containing the first version of the statements was made available via a secure server to the members of the scientific board, who were asked to express their agreement or disagreement on each statement using a 5-point Likert scale (1, strongly disagree; 2, disagree; 3, undecided; 4, agree; 5, strongly agree). Positive consensus was achieved when the proportion of voters selecting items 4 and 5 was ≥ 80%.
In December 2016, a plenary BRIDGE meeting was organized to further discuss the statements and the supporting literature and get feedback from a wider audience of clinicians composed of rheumatologists, dermatologists and gastroenterologists selected to represent IMID management over the entire national territory. To improve discussion, the clinicians were first subdivided into interdisciplinary groups to discuss the statements with the scientific board and the bibliographic fellows and asked to express their agreement or disagreement afterward in a voting session held during the meeting, using an iPad application. The results of the voting were not part of the Delphi process and were collected only to investigate the opinion of a wider audience of clinicians.
Phase 3
In this phase, the results of the first online Delphi voting were evaluated and made available online via a secure server to all scientific board members, along with the results of the survey held during the plenary BRIDGE meeting. Each member was asked to evaluate the results. Based on comments emerging from the discussions held at the BRIDGE meeting and on the results of the first Delphi round, the statements were further refined, with minor changes. The final version of the statements was then submitted to a second Delphi round, performed online as in the first round and involving all members of the scientific board, to confirm the achievement of consensus on all statements.
Results and Discussion
A positive consensus among the experts was reached regarding almost all statements following the first Delphi round. No consensus was reached on two statements concerning the composition of the multidisciplinary team (75% of the experts agreed, 15% were uncertain, and 10% disagreed on the inclusion of ophthalmologists in the IMID team; 75% agreed, 20% were uncertain, and 5% disagreed on the need to provide psychologic support to IMID patients). Based on a discussion among the panel members, the wording of a few statements was changed for the sake of clarity and to reduce redundancies. No changes to the meaning of the statements were made. After the second Delphi round, full positive consensus was reached on all statements. The only statement for which the positive consensus was not full (100%) was that concerning the composition of the IMID team, with 5% of the experts disagreeing on each one of the items related to this issue and 5–10% being uncertain about them. Questions and statements are shown in Table 1, along with the agreement level reached after the second Delphi round.
In the following sections, we present the evidence that led to the formulation of each statement and to the final consensus. We are aware that relevant data may have been published after the last update of the literature searches (October 2016) and that this may constitute an important limitation of our study. This study may also be limited in terms of evidence supporting the effectiveness of the multidisciplinary approach (i.e., few published articles and especially in the dermatologic-rheumatologic field for PsA-Pso). However, the current work, in addition to the evidence in the literature, has also used an expert-based approach and emphasizes the importance of the multidisciplinary approach in stimulating a broader application that may eventually lead to more studies being published on the subject.
Rationale for a Multidisciplinary Approach to SpA-Related IMID Management (Statements 1a, 1b, 1c)
A large body of evidence from epidemiologic studies indicates that patients with chronic inflammatory conditions including Pso, PsA, SpA and IBD are frequently affected by other IMIDs. Up to 30% of patients with Pso are also affected by PsA [20, 21]. According to the results of a recent meta-analysis, ~ 10–15% of patients with Pso have undiagnosed PsA [22]. A retrospective study analyzing 2006–2010 data from the UK Clinical Practice Research Datalink including patients with Pso (n = 27,672) and PsA (n = 1952) has shown that patients with severe Pso have significantly higher rates of comorbidities, including arthritis, than those with mild Pso; patients with PsA have significantly higher rates of arthritis and ankylosing spondylitis than those with severe Pso [23]. The coexistence of several IMIDs is also widely documented for SpA and in particular ankylosing spondylitis. According to a meta-analysis of 143 studies reporting the prevalence of uveitis, Pso and IBD in patients with ankylosing spondylitis (n = 44,372), the pooled prevalences of these conditions were 25.8%, 9.3% and 6.8%, respectively [24]. As for the coexistence of various IMIDs in patients with IBD, a recent study involving a European cohort of 1145 IBD patients followed up for 10 years revealed a greater likelihood that patients with CD would develop extra-intestinal manifestations versus those with UC (20.1% vs. 10.4%, p < 0.001) [25]. Arthritis was the most frequently reported extra-intestinal manifestation (12.9% vs. 8.1%, p = 0.01). The fact that CD patients are significantly more affected than UC patients by extra-intestinal manifestations was also reported by others [26]. Finally, a recent meta-analysis including 71 studies reporting the prevalence of SpA in IBD patients found that the pooled prevalence was 13% for arthritis, 10% for sacroiliitis and 3% for spondylitis [27].
The coexistence of IMIDs in the same patient and within families can be explained by the fact that they have a common genetic background and share pathogenetic mechanisms [1,2,3,4, 28]. The role of the human leukocyte antigen (HLA) B27 in conferring susceptibility to ankylosing spondylitis and other IMIDs is well established, although the mechanistic details of how HLA-B27 mediates joint inflammation have not been fully elucidated [29, 30]. Perhaps the most striking evidence of the overlapping pathophysiology of IMIDs comes from the clinical efficacy of biologic therapies targeting the same inflammatory pathway in a variety of IMIDs. This is reflected in the wide spectrum of IMID indications for TNF-α inhibitors. For example, adalimumab, a monoclonal antibody against TNF-α, is effective in RA, PsA, Pso (plaque psoriasis), IBD (CD, UC, pediatric CD and intestinal Behçet’s disease), ankylosing spondylitis, non-radiographic axial SpA and juvenile idiopathic arthritis [28].
Role of the Multidisciplinary Team in the Implementation of Integrated SpA-Related IMID Management (Statement 2)
Recent literature suggests multiple roles for the multidisciplinary team including: improving diagnosis, optimizing treatment on an in- and outpatient basis, and enhancing patient satisfaction and involvement in their own care [31]. A multidisciplinary approach to patients with moderate-to-severe PsA was associated with improved treatment of skin and joint symptoms and a higher level of patient satisfaction, despite long waiting times [32]. According to the experience from a US combined dermatology and rheumatology clinic in the management of Pso and PsA, almost half of the more than 500 patients referred to the clinic during a 6-year period received a revised diagnosis that was different from the diagnosis received at other centers [33]. Compared with before they attended the combined clinic, patients were more likely to be prescribed systemic medication and biologics. Similar results were reported by a Spanish study describing the 4-year experience of a new multidisciplinary Pso and PsA unit [34]. The multidisciplinary strategy improved diagnosis and symptom control and facilitated early diagnosis of SpA and timely treatment initiation [34]. Preliminary data from the experience of an Italian center with the integrated management of 145 patients with PsA by a dermatologist and a rheumatologist are also promising [35]. A positive impact on disease activity and quality of life (QoL) was reported after only 12 weeks of integrated management.
Impact of the Multidisciplinary Team on All Stages of SpA-Related IMID Management from Presentation to Long-Term Follow-Up (Statement 3)
Multidisciplinary care should be provided from the earliest steps of patient management. In patients with Pso, for example, the early detection of comorbidities is increasingly recognized as a crucial step in patient management. The 4-year experience of the Spanish PSORD multidisciplinary model that operates according to well-defined referral criteria has shown that this approach enables early diagnosis of PsA and subsequent timely treatment initiation [34]. A positive impact of a multidisciplinary care program on the timely initiation of intensive therapy has also been reported in a study comparing an integrated care program with the current standard of care in patients with early RA [36]. Regarding the role of the multidisciplinary approach during treatment and follow-up, a prospective study evaluating medical outcomes in 20 patients with RA receiving multidisciplinary management showed that all patients reported significant improvements in QoL after 3 and 6 months [37] and that this effect was sustained.
Outcome Measures for the Multidisciplinary Approach (Statement 4)
Developing and selecting adequate outcome measures of the effectiveness of integrated IMID care are challenging. In RA, the context in which multidisciplinary team care has been most extensively investigated, the use of function-specific and patient-related outcomes is recommended [38]. In a 10-year study, 55 patients with early RA were treated with a multidisciplinary approach including early and active use of disease-modifying anti-rheumatic drugs (DMARDs), outcome measures included HR-QoL (NHP), disease activity (DAS28), function (HAQ) and joint destruction (Larsen scores) [39]. Overall, all NHP dimensions except social isolation improved significantly during the first 6 months and remained favorable up to 10 years. Early improvements in HR-QoL were sustained over the 10-year observation period for patients with recent-onset RA treated with a multidisciplinary strategy that included early intensive DMARD therapy. A randomized controlled study in 46 patients with ankylosing spondylitis was conducted to compare a 3-week in-patient multidisciplinary rehabilitation program with conventional care [40]. Primary outcomes were disease activity measured with the BASDAI and function measured with the BASFI; secondary outcomes included well-being, spinal and hip mobility and HR-QoL measured with the SF-36. The 3-week multidisciplinary rehabilitation program was found to be more effective than conventional treatment in most outcomes considered.
Goals of the Multidisciplinary Team (Statement 5)
A large body of evidence from studies in patients with psoriatic disease suggests that one of the goals of the multidisciplinary approach is the early diagnosis of the IMIDs that coexist with the disease that has been diagnosed first. As Pso precedes PsA in 75–80% of patients, dermatologists clearly play a key role in the early diagnosis of PsA, and the collaboration among dermatologists, radiologists and rheumatologists is crucial [41, 42]. Evidence shows that prompt initiation of treatment for PsA in patients with Pso can prevent the progression of joint damage and functional disability [43, 44]. Even a 6-month delay from articular symptom onset to the first visit to the rheumatologist contributes to the development of peripheral joint erosions and worse functional impairment [45]. The importance of cooperation between dermatologists and rheumatologists needs to be emphasized to improve the early detection of PsA and enable timely initiation of adequate treatment [46, 47].
Another important goal of the multidisciplinary team is to improve patient-physician communication. In the IBD setting, there is a generally recognized need to improve communication between patients and physicians especially regarding QoL and new treatment options [48]. The patient-physician relationship has been identified as one of the factors that affects adherence to prescribed medication, which is a common problem in UC [49, 50]. In particular, the patient-physician relationship appears to be critical in encouraging adherence through patient education, open communication and agreement on the value of the prescribed treatment [50].
Tools for Achieving the Goals (Statement 6)
In the setting of psoriatic disease, considerable effort has been devoted in recent years to the development of screening tools for the early diagnosis of PsA in patients with Pso, and validated PsA screening tools are available. Four validated screening tools have been recently compared in Pso patients [ToPAS II (Toronto Psoriatic Arthritis Screen II), PASE (Psoriatic Arthritis Screening and Evaluation), PEST (Psoriasis Epidemiology Screening Tool) and EARP (Early Arthritis for Psoriatic Patients] [51]. EARP was found to have the most sensitivity while ToPAS the most specificity. The NICE guidelines recommend the annual use of PEST on all Pso patients without PsA [52, 53]; in the authors’ experience, PEST and EARP are the most useful and practical screening tools for PsA. Magnetic resonance imaging (MRI) and ultrasonography are other useful tools for the early diagnosis of PsA [54].
In the field of CD, recent efforts have been devoted to the identification of signs and symptoms (red flags) that should warn clinicians about the presence of this condition [55, 56]. A Red Flag index has been recently developed to improve early diagnosis of CD, but as yet it has not been validated [55].
Interesting new tools are available using mobile internet technologies. For example, a mobile internet-support service (t-RAppen) has been recently developed to improve self-management of physical activity in RA patients [57].
In recent years, patient-reported outcomes (PROs) have attracted interest as potential assessment tools. A recent study has evaluated alerts generated by a PRO measure-based algorithm for monitoring RA patients and found that the use of the algorithm to screen scheduled visits reduces the chance of missing patients in need of medication intensification [58]. A 7-year follow-up study has evaluated PROs as assessment tools and predictors of long-term prognosis in patients with RA [59]. However, the use of EULAR criteria including PRO for remission is controversial. The study found that the criteria are stringent but important to achieve sustained remission in RA.
When conventional radiography fails to provide conclusive information, MRI techniques are useful for the differential diagnosis, for example, in detecting early stage RA and PsA in the small hand and foot joints [60]. For the evaluation of hand and feet joints and surrounding soft tissue structures in RA and PsA, ultrasound and other imaging techniques are also useful [61].
The first EULAR recommendations for the use of imaging in the diagnosis and management of SpA were published in 2015 [62]. The recommendations encompass the entire spectrum of SpA and evaluate the full role of commonly used imaging techniques, namely: conventional radiography, ultrasound, MRI, computed tomography (CT), positron emission tomography, single-photon emission CT, dual-emission X-ray absorptiometry and scintigraphy. The European Crohn’s and Colitis Organisation (ECCO) recommends upper gastrointestinal endoscopy in the assessment of pediatric and adolescent IBD for classification purposes, and in general endoscopy is recommended to confirm diagnosis and in cases where management needs to be changed [63]. The ECCO guidelines on imaging techniques in IBD state that radiologic imaging techniques complement endoscopic assessment and can assist in the detection and staging of CD [64]; in addition, they state that imaging should be used at first diagnosis for staging and to monitor follow-up. Imaging techniques recommended for IBD include ultrasound, CT, MRI and scintigraphy with radiolabeled leukocytes [64]. Barium contrast and plain film radiology can also be used; however, the use of plain-film radiography is decreasing in favor of ultrasound and CT [64].
Specialists to Be Included in the IMID Team (Statements 7a, 7b, 7c, 7d, 7e)
Direct evidence supporting the inclusion of an ophthalmologist in the multidisciplinary team managing IMIDs is currently lacking. However, the high prevalence of uveitis in IMID patients justifies the presence of an ophthalmologist in the multidisciplinary team to manage eye disease manifestations that can be very bothersome [65, 66]. A study from the Spanish AQUILEA cohort estimated a 2-year incidence of new uveitis cases in patients with SpA of 3.1%, predominantly in patients with ankylosing spondylitis [67]. Conversely, it has been estimated that approximately 40% of patients presenting with idiopathic acute anterior uveitis have undiagnosed SpA [68]. An algorithm for the assessment of patients with acute anterior uveitis has been recently developed to assist ophthalmologists in the referral of patients to rheumatology clinics for early SpA diagnosis [68].
The literature documenting psychologic distress in IMIDs is extensive [69,70,71,72]. Psychologic distress in RA patients has a negative effect on pain outcomes [71, 73] and has been associated with poor adherence to treatment [74,75,76]. A few studies, mostly in RA and Pso, have evaluated the impact of psychologic assistance on outcomes, with mixed results [77, 78].
International guidelines recommend the involvement of general practitioners (GPs) in the RA multidisciplinary team [9]. Various studies have investigated the relationship between GPs and rheumatologists regarding the referral of patients with inflammatory rheumatic diseases from primary care to rheumatology clinics [79,80,81,82]. According to the results of a survey among Flemish GPs, decisions about intensive treatment initiation in early RA should be made by rheumatologists [79]. Reported barriers to intensive treatment initiation included patients’ resistance and non-adherence, lack of GP involvement and unsatisfactory collaboration with rheumatology services. A study assessing the knowledge of SpA among GPs showed that GPs are aware of classic features of ankylosing spondylitis [81, 82]. However, knowledge about the disease spectrum is limited, and early detection is rare. Addressing these issues in training programs may improve recognition of SpA in primary care.
The role of nurses in multidisciplinary team care of IMIDs has been investigated extensively, with most studies being in the field of chronic rheumatic diseases [83,84,85,86,87,88,89,90,91,92]. A Delphi consensus statement on multidisciplinary teams in IBD recommended the inclusion of an IBD nurse in such a team [31], and in general the inclusion of nurses in the IMID multidisciplinary team is widely recommended [9, 93]. However, the position of nurses within the multidisciplinary team varies markedly depending on the country. In addition, healthcare providers corresponding to the “nurse practitioner” of the UK or US healthcare systems do not exist in many countries, including Italy. As a consequence, many of the data and recommendations present in the literature about the role of nurses in multidisciplinary care of IMID may not be generally applicable. Recommendations defining the role of nurses in addressing unmet needs in the management of RA have been published [94]. The evidence shows that nurses usually spend more time with patients than doctors do; they also engage more in the socio-emotional process of establishing a relationship with the patient [92]. Therefore, nurses are in a unique position to explore patient needs and address unmet needs, educate about treatment and self-injection techniques, monitor safety and progress, and coordinate treatment within the multidisciplinary setting [94]. The role of nurses in the education of psoriatic patients about healthier lifestyle habits that can reduce the risk of metabolic complications associated with Pso has also been suggested [95].
Conclusions
It is interesting to note that the 2009 NICE guidelines for the treatment of RA, while recognizing the lack of supporting evidence, recommend multidisciplinary team care because of the services it can provide to patients with RA [9]. The NICE guidelines also underline patients’ perception of non-clinician members of the team (e.g., specialist nurses) as having more time for them [9]. Also lacking are guidelines recommending IMID-specific models of multidisciplinary management.
A recent survey among Spanish dermatologists and rheumatologists, who provided multidisciplinary care for patients with PsA, investigated different models [96]. Two essential characteristics for the implementation of interdisciplinary model were identified: involvement and empathy of team members and well-defined referral criteria. In the present Delphi process, all of the experts involved agreed about the related nature of IMIDs and the existence of a high prevalence of multiple IMIDs in a single patient. One hundred percent agreement was also obtained regarding the fact that a multidisciplinary approach can result in a more extensive evaluation of diseases and a more comprehensive approach to treatment. It was unanimously agreed that the goals of a multidisciplinary team for SpA-related IMIDs should be to increase the diagnosis of concomitant IMIDs to improve the decision-making process during management and to increase patient satisfaction and adherence to treatment. Early referral and diagnosis, early recognition of concomitant IMIDs and optimizing treatment to improve patient QoL are some of the advantages of using multidisciplinary teams for SpA-related IMIDs. To be effective, the expert panel agreed that a multidisciplinary team should be equipped with the appropriate tools for diagnosis and follow-up and at a minimum the multidisciplinary team should include a dermatologist, gastroenterologist and rheumatologist; high agreement (90–95%) was also obtained regarding the importance of providing psychologic support via a psychologist and involving an ophthalmologist, GPs and nurses in multidisciplinary care.
We are aware that evidence supporting the effectiveness of the strategy used here is limited as only a few published studies have specifically assessed the impact of the multidisciplinary approach in terms of diagnostic and therapeutic improvement. Nonetheless, the current study has incorporated data from the literature (i.e., systematic research, therefore evidence based) with the opinion of experts (i.e., specialists of various disciplines with appropriate scientific background and direct clinical experience in multidisciplinary management). Importantly, this is the first study that has analyzed the advantages of the multidisciplinary approach from a double point of view (evidence-based plus personal experience) and that aims to spread the application for a better management of complex pathologies such as SpA-related IMIDs. Future multicenter studies with extensive case studies that specifically evaluate the impact of the multidisciplinary approach are needed. It would also be of interest to evaluate the advantages of the multidisciplinary approach in terms of economic benefits (i.e., early diagnosis and therefore lower disability, appropriate use of diagnostic methods, appropriate and shared use by more specialists of high-cost drugs).
References
Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345(5):340–50.
Kuek A, Hazleman BL, Ostor AJ. Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Postgrad Med J. 2007;83(978):251–60.
Brophy S, Pavy S, Lewis P, et al. Inflammatory eye, skin, and bowel disease in spondyloarthritis: genetic, phenotypic, and environmental factors. J Rheumatol. 2001;28(12):2667–73.
Blandizzi C, Gionchetti P, Armuzzi A, et al. The role of tumour necrosis factor in the pathogenesis of immune-mediated diseases. Int J Immunopathol Pharmacol. 2014;27(1 Suppl):1–10.
Canete JD, Dauden E, Queiro R, et al. Recommendations for the coordinated management of psoriatic arthritis by rheumatologists and dermatologists: a Delphi study. Actas Dermosifiliogr. 2014;105(3):216–32.
Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510.
Olivieri I, Cantini F, Castiglione F, et al. Italian Expert Panel on the management of patients with coexisting spondyloarthritis and inflammatory bowel disease. Autoimmun Rev. 2014;13(8):822–30.
van Erp SJ, Brakenhoff LK, van Gaalen FA, et al. Classifying back pain and peripheral joint complaints in inflammatory bowel disease patients: a prospective longitudinal follow-up study. J Crohn’s Colitis. 2016;10(2):166–75.
National Institute for Health and Care Excellence. Rheumatoid arthritis in adults: management. 2009. http://www.nice.org.uk/guidance/cg79/resources/rheumatoid-arthritis-in-adults-management-pdf-975636823525. Accessed 29 Jun 2017.
Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–71.
van der Heijde D, Ramiro S, Landewe R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–91.
Bearne LM, Byrne AM, Segrave H, White CM. Multidisciplinary team care for people with rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol Int. 2016;36(3):311–24.
Crossland V, Field R, Ainsworth P, Edwards CJ, Cherry L. Is there evidence to support multidisciplinary healthcare working in rheumatology? A systematic review of the literature. Musculoskeletal Care. 2015;13(1):51–66.
Louis E, Dotan I, Ghosh S, Mlynarsky L, Reenaers C, Schreiber S. Optimising the inflammatory bowel disease unit to improve quality of care: expert recommendations. J Crohn’s Colitis. 2015;9(8):685–91.
Migliore A, Cusano F, Bianchi G, Malara G, Epis O, De Pita O. Management of psoriatic arthritis: should the interaction between dermatologists and rheumatologists in clinical practice be intensified? J Biol Regul Homeost Agents. 2015;29(3):547–61.
Vliet Vlieland TP, Li LC, MacKay C, Badley EM. Does everybody need a team? J Rheumatol. 2006;33(9):1897–9.
Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–15.
Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–80.
Powell C. The Delphi technique: myths and realities. J Adv Nurs. 2003;41(4):376–82.
Henes JC, Ziupa E, Eisfelder M, et al. High prevalence of psoriatic arthritis in dermatological patients with psoriasis: a cross-sectional study. Rheumatol Int. 2014;34(2):227–34.
Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–35.
Villani AP, Rouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73(2):242–8.
Edson-Heredia E, Zhu B, Lefevre C, et al. Prevalence and incidence rates of cardiovascular, autoimmune, and other diseases in patients with psoriatic or psoriatic arthritis: a retrospective study using Clinical Practice Research Datalink. J Eur Acad Dermatol Venereol. 2015;29(5):955–63.
Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(1):65–73.
Isene R, Bernklev T, Hoie O, et al. Extraintestinal manifestations in Crohn’s disease and ulcerative colitis: results from a prospective, population-based European inception cohort. Scand J Gastroenterol. 2015;50(3):300–5.
Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol. 2014;20(46):17463–7.
Karreman MC, Luime JJ, Hazes JMW, Weel A. The prevalence and incidence of axial and peripheral spondyloarthritis in inflammatory bowel disease: a systematic review and meta-analysis. J Crohn’s Colitis. 2017;11(5):631–42.
Lapadula G, Marchesoni A, Armuzzi A, et al. Adalimumab in the treatment of immune-mediated diseases. Int J Immunopathol Pharmacol. 2014;27(1 Suppl):33–48.
D’Ambrosio EM, La Cava M, Tortorella P, Gharbiya M, Campanella M, Iannetti L. Clinical features and complications of the HLA-B27-associated acute anterior uveitis: a metanalysis. Semin Ophthalmol. 2017;32(6):689–701.
Salvarani C, Fries W. Clinical features and epidemiology of spondyloarthritides associated with inflammatory bowel disease. World J Gastroenterol. 2009;15(20):2449–55.
Calvet X, Panes J, Alfaro N, et al. Delphi consensus statement: quality indicators for inflammatory bowel disease comprehensive care units. J Crohn’s Colitis. 2014;8(3):240–51.
Cobo-Ibanez T, Villaverde V, Seoane-Mato D, et al. Multidisciplinary dermatology-rheumatology management for patients with moderate-to-severe psoriasis and psoriatic arthritis: a systematic review. Rheumatol Int. 2016;36(2):221–9.
Velez NF, Wei-Passanese EX, Husni ME, Mody EA, Qureshi AA. Management of psoriasis and psoriatic arthritis in a combined dermatology and rheumatology clinic. Arch Dermatol Res. 2012;304(1):7–13.
Luelmo J, Gratacos J, Moreno Martinez-Losa M, et al. A report of 4 years of experience of a multidisciplinary unit of psoriasis and psoriatic arthritis. Reumatol Clin. 2014;10(3):141–6.
Capeci W, Luchetti MM, Campanati A, et al. The multidisciplinary approach is the best option to achieve the improvement of disease activity and quality of life in patients affected by psoriatic arthritis: preliminary results of a monocentric study [Abstract THU0454]. Ann Rheum Dis. 2016;75(Suppl 2):356.
Esselens G, Westhovens R, Verschueren P. Effectiveness of an integrated outpatient care programme compared with present-day standard care in early rheumatoid arthritis. Musculoskeletal care. 2009;7(1):1–16.
Maravic M, Bozonnat MC, Sevezan A, et al. Preliminary evaluation of medical outcomes (including quality of life) and costs in incident RA cases receiving hospital-based multidisciplinary management. Jt Bone Spine. 2000;67(5):425–33.
Vliet Vlieland TP. Multidisciplinary team care and outcomes in rheumatoid arthritis. Curr Opin Rheumatol. 2004;16(2):153–6.
Uutela T, Hannonen P, Kautiainen H, Hakala M, Hakkinen A. Sustained improvement of health-related quality of life in patients with early rheumatoid arthritis: a ten-year follow-up study. Clin Exp Rheumatol. 2011;29(1):65–71.
Kjeken I, Bo I, Ronningen A, et al. A three-week multidisciplinary in-patient rehabilitation programme had positive long-term effects in patients with ankylosing spondylitis: randomized controlled trial. J Rehabil Med. 2013;45(3):260–7.
Garg A, Gladman D. Recognizing psoriatic arthritis in the dermatology clinic. J Am Acad Dermatol. 2010;63(5):733–48.
Weger W. An update on the diagnosis and management of psoriatic arthritis. G Ital Dermatol Venereol. 2011;146(1):1–8.
Radtke MA, Mrowietz U, Feuerhahn J, et al. Early detection of comorbidity in psoriasis: recommendations of the National Conference on Healthcare in Psoriasis. Journal der Deutschen Dermatologischen Gesellschaft J Ger Soc Dermatol JDDG. 2015;13(7):674–90.
Radtke MA, Reich K, Blome C, Rustenbach S, Augustin M. Prevalence and clinical features of psoriatic arthritis and joint complaints in 2009 patients with psoriasis: results of a German national survey. J Eur Acad Dermatol Venereol. 2009;23(6):683–91.
Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1045–50.
Olivieri I, D’Angelo S, Palazzi C, Padula A. Advances in the management of psoriatic arthritis. Nat Rev Rheumatol. 2014;10(9):531–42.
Richard MA, Barnetche T, Rouzaud M, et al. Evidence-based recommendations on the role of dermatologists in the diagnosis and management of psoriatic arthritis: systematic review and expert opinion. J Eur Acad Dermatol Venereol. 2014;28(Suppl 5):3–12.
Mitchell R, Kremer A, Westwood N, Younge L, Ghosh S. Talking about life and IBD: a paradigm for improving patient-physician communication. J Crohn’s Colitis. 2009;3(1):1–3.
Kane SV, Brixner D, Rubin DT, Sewitch MJ. The challenge of compliance and persistence: focus on ulcerative colitis. J Manag Care Pharm. 2008;14(1):s2–12 (quiz s13-5).
Lakatos PL. Prevalence, predictors, and clinical consequences of medical adherence in IBD: how to improve it? World J Gastroenterol. 2009;15(34):4234–9.
Mishra S, Kancharla H, Dogra S, Sharma A. Comparison of four validated psoriatic arthritis screening tools in diagnosing psoriatic arthritis in patients with psoriasis (COMPAQ Study). Br J Dermatol. 2017;176(3):765–70.
Ganatra B, Manoharan D, Akhras V. Use of a validated screening tool for psoriatic arthritis in dermatology clinics. BMJ Qual Improv Rep. 2015;4(1):u203335.w2644. https://doi.org/10.1136/bmjquality.u203335.w2644
National Institute for Health and Care Excellence. Psoriasis: assessment and management. 2012. http://www.nice.org.uk/guidance/cg153/resources/psoriasis-assessment-and-management-35109629621701. Accessed 29 Jun 2017.
D’Angelo S, Palazzi C, Gilio M, Leccese P, Padula A, Olivieri I. Improvements in diagnostic tools for early detection of psoriatic arthritis. Expert Rev Clin Immunol. 2016;12(11):1209–15.
Danese S, Fiorino G, Mary JY, et al. Development of red flags index for early referral of adults with symptoms and signs suggestive of Crohn’s disease: an IOIBD initiative. J Crohn’s Colitis. 2015;9(8):601–6.
Fiorino G, Danese S. Diagnostic delay in Crohn’s disease: time for red flags. Dig Dis Sci. 2016;61(11):3097–8.
Revenas A, Opava CH, Ahlen H, Brusewitz M, Pettersson S, Asenlof P. Mobile internet service for self-management of physical activity in people with rheumatoid arthritis: evaluation of a test version. RMD Open. 2016;2(1):e000214.
Hendrikx J, Fransen J, van Riel PL. Monitoring rheumatoid arthritis using an algorithm based on patient-reported outcome measures: a first step towards personalised healthcare. RMD open. 2015;1(1):e000114.
Kojima M, Kojima T, Suzuki S, et al. Patient-reported outcomes as assessment tools and predictors of long-term prognosis: a 7-year follow-up study of patients with rheumatoid arthritis. Int J Rheum Dis. 2017;20(9):1193–200.
Jevtic V, Lingg G. Differential diagnosis of rheumatoid and psoriatic arthritis at an early stage in the small hand and foot joints using magnetic resonance imaging. Handchir Mikrochir Plast Chir. 2012;44(3):163–70.
Gutierrez M, Filippucci E, Salaffi F, Di Geso L, Grassi W. Differential diagnosis between rheumatoid arthritis and psoriatic arthritis: the value of ultrasound findings at metacarpophalangeal joints level. Ann Rheum Dis. 2011;70(6):1111–4.
Mandl P, Navarro-Compan V, Terslev L, et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann Rheum Dis. 2015;74(7):1327–39.
Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohn’s Colitis. 2013;7(12):982–1018.
Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohn’s Colitis. 2013;7(7):556–85.
Abbouda A, Abicca I, Fabiani C, et al. Psoriasis and psoriatic arthritis-related uveitis: different ophthalmological manifestations and ocular inflammation features. Semin Ophthalmol. 2017;32(6):715–20.
Generali E, Cantarini L, Selmi C. Ocular involvement in systemic autoimmune diseases. Clin Rev Allergy Immunol. 2015;49(3):263–70.
Garcia-Vicuna R, Zarco P, Gonzalez CM, Vanaclocha F, Marin-Jimenez I, Cea-Calvo L. Two-year incidence of psoriasis, uveitis and inflammatory bowel disease in patients with spondyloarthritis: a study in the AQUILES cohort. Reumatol Clin. 2016;12(1):22–6.
Haroon M, O’Rourke M, Ramasamy P, Murphy CC, FitzGerald O. A novel evidence-based detection of undiagnosed spondyloarthritis in patients presenting with acute anterior uveitis: the DUET (Dublin Uveitis Evaluation Tool). Ann Rheum Dis. 2015;74(11):1990–5.
Bacconnier L, Rincheval N, Flipo RM, et al. Psychological distress over time in early rheumatoid arthritis: results from a longitudinal study in an early arthritis cohort. Rheumatology (Oxford). 2015;54(3):520–7.
Offidani E, Del Basso D, Prignago F, Tomba E. Discriminating the presence of psychological distress in patients suffering from psoriasis: an application of the clinimetric approach in dermatology. Acta Derm Venereol. 2016;96(217):69–73.
Ryan S, McGuire B. Psychological predictors of pain severity, pain interference, depression, and anxiety in rheumatoid arthritis patients with chronic pain. Br J Health Psychol. 2016;21(2):336–50.
Sturgeon JA, Finan PH, Zautra AJ. Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways. Nat Rev Rheumatol. 2016;12(9):532–42.
Knowles SR, Wilson JL, Connell WR, Kamm MA. Preliminary examination of the relations between disease activity, illness perceptions, coping strategies, and psychological morbidity in Crohn’s disease guided by the common sense model of illness. Inflamm Bowel Dis. 2011;17(12):2551–7.
Morgan C, McBeth J, Cordingley L, et al. The influence of behavioural and psychological factors on medication adherence over time in rheumatoid arthritis patients: a study in the biologics era. Rheumatology (Oxford). 2015;54(10):1780–91.
Tabibian A, Tabibian JH, Beckman LJ, Raffals LL, Papadakis KA, Kane SV. Predictors of health-related quality of life and adherence in Crohn’s disease and ulcerative colitis: implications for clinical management. Dig Dis Sci. 2015;60(5):1366–74.
Vangeli E, Bakhshi S, Baker A, et al. A systematic review of factors associated with non-adherence to treatment for immune-mediated inflammatory diseases. Adv Ther. 2015;32(11):983–1028.
Chen Y, Xin T, Cheng AS. Evaluating the effectiveness of psychological and/or educational interventions in psoriasis: a narrative review. J Dermatol. 2014;41(9):775–8.
Dissanayake RK, Bertouch JV. Psychosocial interventions as adjunct therapy for patients with rheumatoid arthritis: a systematic review. Int J Rheum Dis. 2010;13(4):324–34.
Meyfroidt S, Stevens J, De Lepeleire J, et al. A general practice perspective on early rheumatoid arthritis management: a qualitative study from Flanders. Eur J Gen pract. 2015;21(4):231–7.
Puchner R, Edlinger M, Mur E, et al. Interface management between general practitioners and rheumatologists-results of a survey defining a concept for future joint recommendations. PLoS One. 2016;11(1):e0146149.
van Onna M, Gorter S, Maiburg B, Waagenaar G, van Tubergen A. Education improves referral of patients suspected of having spondyloarthritis by general practitioners: a study with unannounced standardised patients in daily practice. RMD open. 2015;1(1):e000152.
van Onna M, Gorter S, van Meerendonk A, van Tubergen A. General practitioners’ perceptions of their ability to identify and refer patients with suspected axial spondyloarthritis: a qualitative study. J Rheumatol. 2014;41(5):897–901.
Amity CL, Schlenk EA, Gold KN, et al. Agreement of physicians and nurses performing tender and swollen joint counts in rheumatoid arthritis. J Clin Rheumatol. 2016;22(1):30–4.
Bala SV, Samuelson K, Hagell P, Svensson B, Fridlund B, Hesselgard K. The experience of care at nurse-led rheumatology clinics. Musculoskeletal Care. 2012;10(4):202–11.
Candelas G, Villaverde V, Garcia S, Guerra M, Leon MJ, Canete JD. Benefit of health education by a training nurse in patients with axial and/or peripheral psoriatic arthritis: a systematic literature review. Rheumatol Int. 2016;36(11):1493–506.
Fall E, Chakroun N, Dalle N, Izaute M. Is patient education helpful in providing care for patients with rheumatoid arthritis? A qualitative study involving French nurses. Nurs Health Sci. 2013;15(3):346–52.
Jelsness-Jorgensen LP, Bernklev T, Henriksen M, Torp R, Moum B. Is patient reported outcome (PRO) affected by different follow-up regimens in inflammatory bowel disease (IBD)? A one year prospective, longitudinal comparison of nurse-led versus conventional follow-up. J Crohn’s Colitis. 2012;6(9):887–94.
Munoz-Fernandez S, Aguilar MD, Rodriguez A, et al. Evaluation of the impact of nursing clinics in the rheumatology services. Rheumatol Int. 2016;36(9):1309–17.
O’Connor M, Gaarenstroom J, Kemp K, Bager P, van der Woude CJ. N-ECCO survey results of nursing practice in caring for patients with Crohn’s disease or ulcerative colitis in Europe. J Crohn’s colitis. 2014;8(10):1300–7.
Primdahl J, Sorensen J, Horn HC, Petersen R, Horslev-Petersen K. Shared care or nursing consultations as an alternative to rheumatologist follow-up for rheumatoid arthritis outpatients with low disease activity–patient outcomes from a 2-year, randomised controlled trial. Ann Rheum Dis. 2014;73(2):357–64.
Solomon DH, Fraenkel L, Lu B, et al. Comparison of care provided in practices with nurse practitioners and physician assistants versus subspecialist physicians only: a cohort study of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2015;67(12):1664–70.
Vinall-Collier K, Madill A, Firth J. A multi-centre study of interactional style in nurse specialist- and physician-led rheumatology clinics in the UK. Int J Nurs Stud. 2016;59:41–50.
Larsson I, Fridlund B, Arvidsson B, Teleman A, Svedberg P, Bergman S. A nurse-led rheumatology clinic versus rheumatologist-led clinic in monitoring of patients with chronic inflammatory arthritis undergoing biological therapy: a cost comparison study in a randomised controlled trial. BMC Musculoskelet Disord. 2015;16:354.
Cottrell JE, Jonas M, Bergsten U, et al. The nurse’s role in addressing unmet treatment and management needs of patients with rheumatoid arthritis: Delphi-based recommendations. Int J Nurs Knowl. 2013;24(2):66–76.
Aldridge A. The role of the community nurse in psoriatic comorbidities interventions. Br J Community Nurs. 2014;19(1):38–42.
Queiro R, Coto P, Rodriguez J, et al. Multidisciplinary care models for patients with psoriatic arthritis. Reumatol Clin. 2017;13(2):85–90.
Acknowledgements
Funding
Sponsorship for the Delphi consensus meetings and article processing charges were funded by Aristea and Hippocrates. All authors had full access to all of the data in this manuscript and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published.
Medical Writing, Editorial and Other Assistance
We would like to thank Aristea and Hippocrates for organizing and conducting the methodology of the study and Springer Healthcare for the editorial support in drafting the manuscript. Sheridan Henness, PhD, of Springer Healthcare Communications produced the second draft of this manuscript and edited the manuscript for English language. Melanie Gatt, an independent medical writer on behalf of Springer Healthcare Communications, assisted with language and technical editing of post-submission revision. This assistance was funded by Aristea and Hippocrates.
Disclosures
Fernando Rizzello has served as speaker and consultant and received research grants from AbbVie, Janssen, MSD, Takeda, Pfizer, Ferring, Chiesi and Sofar. Alessandro Armuzzi has served as a consultant or advisory member for AbbVie, Allergan, Biogen, Celltrion, Ferring, Hospira, Janssen, Lilly, MSD, Mundipharma, Pfizer, Samsung, Sofar and Takeda; has received lecture fees from AbbVie, AstraZeneca, Chiesi, Ferring, Hospira, MSD, Mundipharma, Nikkiso, Otsuka, Pfizer, Takeda, Tigenix and Zambon; has received research funding from MSD. Fabio Ayala has served as speaker, consultant and advisory board member for AbbVie, Almirall, Lilly, MSD, Pierre Fabre, Novartis, Pfizer, Sanofi-Genzyme and UCB. Luca Bianchi has served as speaker and consultant for AbbVie, Janssen, Pfizer, Ucb, Celgene, Novartis and Johnson & Johnson. Luca Cimino has served as consultant for AbbVie and Santen. Antonio Costanzo has acted as speaker or received research grants from AbbVie, Janssen, Novartis, Celgene and Pfizer. Salvatore D’Angelo declares consulting fees, research or institutional support and educational grants from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Merck Sharp & Dohme, Novartis, Pfizer and UCB. Marco Daperno received hospital grants (by Takeda), participated on the board or gave lectures (for AbbVie, Janssen, Takeda, MSD, Pfizer, Hospira, SOFAR, Chiesi) or was supported for congress participation (by AbbVie, Janssen, Takeda, MSD, Pfizer, Hospira, Mundipharma, SOFAR, Chiesi, Ferring, Zambon). Paolo Gionchetti received speaker fees from AbbVie, MSD, Takeda, Ferring, Sofar and Chiesi Alfa Wasserman and received honoraria for participation in advisory boards for Janssens, Takeda, MSD, AbbVie, Alfa Wasserman, Celgene, Amgen, Hospira and Pfizer. Paolo Gisondi has served as consultant for AbbVie, Novartis, Eli Lilly, Celgene, UCB, Pfizer, Jansen, MSD and Mundipharma. Ennio Lubrano received consulting and speaker’s honoraria from AbbVie, Celgene, Janssen Cilag, MSD, Pfizer, Novartis and Sanofi-Genzyme. Antonio Marchesoni received consulting or speaker’s honoraria from Abbvie, Pfizer, MSD, Celgene, Janssen, Novartis and BSM. Annamaria Offidani has served as a speaker, advisory board member, consultant for AbbVie, Pfizer, Novartis, Lilly, Galderma and Celgene. Ambrogio Orlando served as advisory board member to AbbVie, Janssen, MSD and Takeda Pharmaceutical and received lecture grants from AbbVie, Chiesi Farmaceutici, MSD, Sofar and Takeda Pharmaceutical. Daniela Pugliese received lecture fees from AbbVie and Takeda. Raffaele Scarpa has served as a speaker, consultant, advisory board member for AbbVie, MSD, Novartis, Pfizer, Jannsen,Celgene and UCB. Maurizio Vecchi received speaker’s fees, participated in advisory boards or received research funding from AbbVie, Biogen, Chiesi, Giuliani, Hospira, Jannsen, MSD, Mundipharma, Sofar, Takeda and Zambon. Giampiero Girolomoni has been principal investigator in clinical trials sponsored by and/or has received personal fees from AbbVie, Abiogen, Almirall, Amgen, Bayer, Biogen, Celgene, Eli-Lilly, Galderma, Genzyme, Hospira, Janssen, Leo Pharma, Menlo therapeutics, Merck, MSD, Mundipharma, Novartis, Pfizer, Pierre Fabre, Regeneron, Samsung, Sandoz, Sanofi, Serono and Sun Pharma. Vincenzo Bettoli, Antonio Cristaudo, Anna Chiara Fostini, Mauro Galeazzi, Michele Gilio, Carlo Salvarani and Ignazio Olivieri have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ignazio Olivieri: Deceased. This manuscript is dedicated to the memory of Ignazio Olivieri who participated in the present manuscript.
Enhanced content
To view enhanced content for this article go to https://doi.org/10.6084/m9.figshare.5868042.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rizzello, F., Olivieri, I., Armuzzi, A. et al. Multidisciplinary Management of Spondyloarthritis-Related Immune-Mediated Inflammatory Disease. Adv Ther 35, 545–562 (2018). https://doi.org/10.1007/s12325-018-0672-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0672-6