Abstract
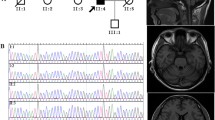
Spinocerebellar ataxia type 3 or Machado-Joseph disease (MJD/SCA3) is the most prevalent autosomal dominant cerebellar ataxia worldwide, but its frequency varies by geographic region. We describe MJD/SCA3 patients diagnosed in a tertiary healthcare institution in Peru. In a cohort of 341 individuals (253 probands) with clinical ataxia diagnosis, seven MJD/SCA3 probands were identified and their pedigrees extended, detecting a total of 18 MJD/SCA3 cases. Out of 506 alleles from all probands from this cohort, the 23-CAG repeat was the most common ATXN3 allele (31.8%), followed by the 14-CAG repeat allele (26.1%). Normal alleles ranged from 12 to 38 repeats while pathogenic alleles ranged from 64 to 75 repeats. We identified 80 large normal (LN) alleles (15.8%). Five out of seven families declared an affected family member traced back to foreign countries (England, Japan, China, and Trinidad and Tobago). MJD/SCA3 patients showed ataxia, accompanied by pyramidal signs, dysarthria, and dysphagia as well as abnormal oculomotor movements. In conclusion, ATXN3 allelic distribution in non-MJD/SCA3 patients with ataxia is similar to the distribution in normal individuals around the world, whereas LN allele frequency reinforces no correlation with the frequency of MJD/SCA3. Evidence of any atypical MJD/SCA3 phenotype was not found. Furthermore, haplotypes are required to confirm the foreign origin of MJD/SCA3 in the Peruvian population.



Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Ichikawa Y, Goto J, Hattori M, Toyoda A, Ishii K, Jeong S-Y, et al. The genomic structure and expression of MJD, the Machado-Joseph disease gene. J Hum Genet. 2001;46:413–22.
Bettencourt C, Lima M. Machado-Joseph Disease: from first descriptions to new perspectives. Orphanet J Rare Dis. 2011;6:35.
Coutinho P, Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands: a new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978;28:703–703.
Moro A, Munhoz RP, Arruda WO, Raskin S, Moscovich M, Teive HAG. Spinocerebellar ataxia type 3: subphenotypes in a cohort of brazilian patients. Arq Neuro-Psiquiatr. 2014;72:659–62.
Moro A, Moscovich M, Farah M, Camargo CHF, Teive HAG, Munhoz RP. Nonmotor symptoms in spinocerebellar ataxias (SCAs). Cerebellum Ataxias. 2019;6:12.
Martins S, Sequeiros J. Origins and spread of Machado-Joseph disease ancestral mutations events. In: Nóbrega C, Pereira de Almeida L, editors. Polyglutamine Disorders [Internet]. Cham: Springer International Publishing; 2018 [cited 2022 Sep 8]. p. 243–54. Available from: http://link.springer.com/10.1007/978-3-319-71779-1_12.
de Castilhos RM, Furtado GV, Gheno TC, Schaeffer P, Russo A, et al. Spinocerebellar ataxias in Brazil—frequencies and modulating effects of related genes. Cerebellum. 2014;13:17–28.
Vale J, Bugalho P, Silveira I, Sequeiros J, Guimarães J, Coutinho P. Autosomal dominant cerebellar ataxia: frequency analysis and clinical characterization of 45 families from Portugal: autosomal dominant cerebellar ataxia: characterization of 45 Portuguese families. Eur J Neurol. 2010;17:124–8.
Brusco A, Gellera C, Cagnoli C, Saluto A, Castucci A, Michielotto C, et al. Molecular genetics of hereditary spinocerebellar ataxia: mutation analysis of spinocerebellar ataxia genes and CAG/CTG repeat expansion detection in 225 Italian families. Arch Neurol. 2004;61:727.
Bryer A, Krause A, Bill P, Davids V, Bryant D, Butler J, et al. The hereditary adult-onset ataxias in South Africa. J Neurol Sci. 2003;216:47–54.
Paradisi I, Ikonomu V, Arias S. Spinocerebellar ataxias in Venezuela: genetic epidemiology and their most likely ethnic descent. J Hum Genet. 2016;61:215–22.
Miranda CM. Diagnóstico de Ataxia Espinocerebelosa tipo 3 (Enfermedad de Machado-Joseph) en Chile. Rev méd Chile. 2015;143:126–7.
Rodríguez-Quiroga SA, Cordoba M, González-Morón D, Medina N, Vega P, Dusefante CV, et al. Neurogenetics in Argentina: diagnostic yield in a personalized research based clinic. Genet Res. 2015;97: e10.
Alonso E, Martínez-Ruano L, De Biase I, Mader C, Ochoa A, Yescas P, et al. Distinct distribution of autosomal dominant spinocerebellar ataxia in the Mexican population. Mov Disord. 2007;22:1050–3.
Velázquez Pérez L, Cruz GS, Santos Falcón N, Enrique Almaguer Mederos L, Escalona Batallan K, Rodríguez Labrada R, et al. Molecular epidemiology of spinocerebellar ataxias in Cuba: insights into SCA2 founder effect in Holguin. Neurosci Lett. 2009;454:157–60.
Torres Ramirez L, Vélez Rojas M, Mazzetti Soler P, Suárez Reyes R, Cosentino Esquerre C, Mori Quispe N et al. Ataxia espinocerebelosa tipo 3 (Enfermedad de Machado Joseph). A propósito de un caso Diagnóstico. 2012;51(1):33–6.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res. 1988;16:1215–1215.
Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8:221–8.
Melo ARV, Ramos A, Kazachkova N, Raposo M, Bettencourt BF, Rendeiro AR, et al. Triplet repeat primed PCR (TP-PCR) in molecular diagnostic testing for spinocerebellar ataxia type 3 (SCA3). Mol Diagn Ther. 2016;20:617–22.
Saute JAM, Jardim LB. Machado Joseph disease: clinical and genetic aspects, and current treatment. Expert Opin Orphan Drugs. 2015;3:517–35.
Takano H, Cancel G, Ikeuchi T, Lorenzetti D, Mawad R, Stevanin G, et al. Close associations between prevalences of dominantly inherited spinocerebellar ataxias with CAG-repeat expansions and frequencies of large normal CAG alleles in Japanese and Caucasian populations. Am J Hum Genet. 1998;63:1060–6.
Cornejo-Olivas M, Castilhos RM, Furtado GV, Mattos EP, Bampi GB, Leistner-Segal S, et al. Genetic analysis of hereditary ataxias in Peru identifies SCA10 families with incomplete penetrance. Cerebellum. 2020;19:208–15.
Teive HAG, Moro A, Arruda WO, Raskin S, Teive GMG, Dallabrida N, et al. Itajaí, Santa Catarina – Azorean ancestry and spinocerebellar ataxia type 3. Arq Neuro-Psiquiatr. 2016;74:858–60.
Rannala B, Mountain JL. Detecting immigration by using multilocus genotypes. Proc Natl Acad Sci USA. 1997;94:9197–201.
Bonfiglio G. Introducción al estudio de la inmigración europea en el Perú. Apuntes. 1986;18:93–127.
Hu-Dehart E. Coolies, shopkeepers, pioneers: the Chinese of Mexico and Peru (1849–1930). Amerasia J. 1989;15:91–116.
Takenaka A. The Japanese in Peru: history of immigration, settlement, and racialization. Lat Am Perspect. 2004;31:77–98.
Moro A, Munhoz RP, Arruda WO, Raskin S, Teive HAG. Clinical relevance of “bulging eyes” for the differential diagnosis of spinocerebellar ataxias. Arq Neuro-Psiquiatr. 2013;71:428–30.
Franklin GL, Meira AT, Camargo CHF, Nascimento FA, Teive HAG. Upward gaze palsy: a valuable sign to distinguish spinocerebellar ataxias. Cerebellum. 2020;19:685–90.
Friedman JH, Fernandez HH, Sudarsky LR. REM behavior disorder and excessive daytime somnolence in Machado-Joseph disease (SCA-3). Mov Disord. 2003;18:1520–2.
Pedroso JL, França MC, Braga-Neto P, D’Abreu A, Saraiva-Pereira ML, Saute JA, et al. Nonmotor and extracerebellar features in Machado-Joseph disease: a review: extracerebellar features in Machado-Joseph disease. Mov Disord. 2013;28:1200–8.
Storey E, du Sart D, Shaw JH, Lorentzos P, Kelly L, McKinley Gardner RJ, et al. Frequency of spinocerebellar ataxia types 1, 2, 3, 6, and 7 in Australian patients with spinocerebellar ataxia. Am J Med Genet. 2000;95:351–8.
Lima M, Costa MC, Montiel R, Ferro A, Santos C, Silva C, et al. Population genetics of wild-type CAG repeats in the Machado-Joseph Disease gene in Portugal. Hum Hered. 2005;60:156–63.
Gan S-R, Ni W, Dong Y, Wang N, Wu Z-Y. Population genetics and new insight into range of CAG repeats of spinocerebellar ataxia type 3 in the Han Chinese population. Li X-J, editor. PLoS One. 2015;10:e0134405.
Gonzales-Sáenz C, Cruz-Rodriguez C, Espinoza-Huertas K, Véliz-Otani D, Marca V, Ortega O, et al. Distribution of the CAG triplet repeat in ATXN1, ATXN3, and CACNA1A loci in Peruvian population. Cerebellum. 2020;19:527–35.
Acknowledgements
We are grateful to Victoria Marca-Ysabel, Miguel Inca-Martínez, and Diego Veliz-Otani for logistic support and lab assistance; Melissa Moleros for her assistance on recruitment of participants; and Lucy Stirland for her review of the manuscript. We are grateful to the DNA-Neurogenetics Bank of the Instituto Nacional de Ciencias Neurológicas for supporting the collection of DNA samples and associated data used in this publication. Samples from the DNA-Neurogenetics Bank were obtained through informed consent and IRB approval. The content in this publication does not reflect the opinion of the DNA-Neurogenetics Bank.
Funding
This study was funded by the Peruvian Institution PROCIENCIA-CONCYTEC within the framework of the convention of Research Projects in Health EU-LAC (Contract No. 098–2017-FONDECYT). Authors affiliated to Instituto National de Ciencias Neurológicas are also partially supported by Contract No. 148–2020-PROCIENCIA.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript. All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Lesly Solis-Ponce, Elison Sarapura-Castro, Karina Milla-Neyra, Maryenela Illanes-Manrique, Pilar Mazzetti, Ismael Araujo-Aliaga, Olimpio Ortega, Carla Manrique-Enciso, Diana Cubas-Montecino, Maria Luiza Saraiva-Pereira, Laura B. Jardim, and Mario Cornejo-Olivas. The first draft of the manuscript was written by Ismael Araujo-Aliaga, Lesly Solis-Ponce, and Mario Cornejo-Olivas, and all authors commented on later versions of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by the Ethics Committee at INCN, called the “Comité Institucional de Ética en Investigación del Instituto Nacional de Ciencias Neurológicas,” IRB number 486–2018-CIEI-INCN. All patients provided written informed consent for use of their genetic and clinical data for anonymized research studies at the time of their genetic testing. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cornejo-Olivas, M., Solis-Ponce, L., Araujo-Aliaga, I. et al. Machado Joseph-Disease Is Rare in the Peruvian Population. Cerebellum 22, 1192–1199 (2023). https://doi.org/10.1007/s12311-022-01491-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01491-4