Abstract
Chronic myeloid leukaemia most commonly presents in chronic phase (CML-CP) and it is characterised by granulocytic proliferation. Many patients have an excellent response to tyrosine kinase inhibitor therapy; however, a small proportion will develop lymphoid or myeloid blast crisis, with inferior clinical outcomes. Detection of lymphoblasts at diagnosis of CML-CP has been reported in small case series with conflicting results on the risk of subsequent blast crisis. The aim of this study was to identify the incidence and immunophenotype of abnormal lymphoblast populations in CML-CP. Retrospective review of bone marrow flow cytometry results of consecutive patients with newly diagnosed CML-CP between June 2012 and February 2021 was performed. Lymphoblasts, myeloblasts, haematogones, and mature lymphocytes were evaluated. Fifty-nine patients had bone marrow flow cytometry results available for review. Abnormal lymphoblast populations were detected in four patients (7%) comprising 0.05–0.19% of bone marrow events. The immunophenotype was similar but distinct from haematogones. The most common distinguishing features of the abnormal lymphoblast populations were abnormally bright expression of CD19 or CD10, weak CD38 or aberrant CD20 expression on CD34 + cells. The clinical case of one of the patients with abnormal lymphoblasts detected at diagnosis who went on to subsequent blast crisis is discussed. Abnormal lymphoblasts can be identified in CML-CP and may be under-recognised. Their detection requires careful analysis in order to distinguish them from normal precursors. The clinical significance of such populations requires further study.
Similar content being viewed by others
Introduction
Chronic myeloid leukaemia (CML) is a myeloproliferative neoplasm originating from a pluripotent haematopoietic stem cell with the potential to differentiate into malignant myeloid and/or lymphoid populations. Typically, CML presents in chronic phase (CML-CP) with granulocytic proliferation, and the majority of patients will have an excellent response to tyrosine kinase inhibitor (TKI) therapy [1]. However, in those who progress to blast phase the prognosis remains poor with 5-year survival of less than 20% in the modern era [2]. The most important predictor of progression is response to TKI therapy. Other factors predicting risk of transformation or death include age, spleen size and basophil count [3,4,5].
Flow cytometry allows for sensitive detection of abnormal haematopoietic populations. As CML-CP is characterised by proliferation of predominantly granulocytes and granulocytic precursors without immunophenotypic aberrancy or maturation arrest, flow cytometry has been considered to be of limited utility in this phase of disease. However, in cases where flow cytometry has been performed, there are reports of detection of abnormal lymphoblast populations with conflicting data on the subsequent risk of development of blast phase [6,7,8,9].
We report here a case of CML-CP with an abnormal lymphoblast population identified at diagnosis. Additionally, a review of cases of CML-CP at our institution was performed with the aim of identifying the incidence and immunophenotype of abnormal lymphoblast populations at diagnosis in patients with CML-CP.
Methods
At our community-based laboratory flow cytometry is performed on all bone marrow specimens. Samples are collected in lithium heparin anticoagulant and analysed within 24 h. Samples are prepared using Beckman coulter FP100, and flow cytometry is performed on the Beckman Coulter Navios flow cytometer. Ten-colour flow using screening tubes to assess myeloid maturation and examine lymphocyte populations (Table 1) is performed with additional analysis as required. Data collection on our antibody tube designed to interrogate myeloid lineage has 2 stops: 100,000 events or 5 min whichever comes first. The tubes designed to interrogate T cell and B cells, respectively, have an additional stop of 5000 events occurring in the “lymphocyte gate” on CD45 versus side scatter.
The case described below was identified through routine analysis, and additional clinical information was provided by the treating clinician with patient consent.
To assess the incidence of abnormal lymphoblast populations in patients with CML-CP, consecutive patients with a BCR-ABL1 fusion detected by RT-PCR with a bone marrow aspirate and trephine performed between June 2012 and February 2021 were identified. Patients were then excluded if they did not have a diagnosis of CML-CP. Flow cytometry of these patients were reviewed by the first and/or last author using Kaluza C version 1.1.
Using our Navios cytometers, with our particular antibody combinations and settings:
Early haematogones are described as CD19weak/ + , CD10 + / + + , CD38 + / + + , CD20-, CD34 + , CD45weak with very low side scatter (SS). Later haematogones are CD19 + , CD10 + , CD38 + / + + , CD20 variable from – to + , CD34-, CD45 + with very low SS. We selected for early haematogones by sequentially gating on CD34 versus SS, then CD19 positivity, ensuring that the events appeared in the correct area on CD45 vs SS. This was correlated with CD19weak, CD10 + / + + , CD38 + / + + events with CD45weak/very low SS on a second analysis. Later haematogones were identified and enumerated by the characteristics above. Aberrant B lymphoblasts were identified by CD19 expressing CD10 ± , surface membrane immunoglobulin (SMIg) negative events of any CD45 intensity that were not plasma cells (CD38 + +) nor abnormal mature B-cells and deviated from the normal maturation pattern described.
A minimum of 20% positive events was used for antigen positivity, and populations comprising less than 20 events were not included. All populations are reported as a percentage of total bone marrow events unless otherwise stated.
Patient outcomes, aside from those in the clinical case, have not been included as we are unable to guarantee the completeness of follow up through our community-based laboratory.
Results
Clinical case
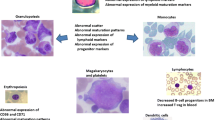
A 73-year-old lady presented with neutrophilia (10.5 × 109/L) and basophilia (1.2 × 109/L) on routine blood tests. BCR/ABL1 dual colour FISH detected the BCR-ABL1 gene fusion (p210 transcript) and bone marrow aspirate and trephine confirmed CML-CP. Flow cytometry on the bone marrow aspirate identified an abnormal lymphoblast population comprising 0.2% of all cells. The phenotype of the lymphoblasts was CD45weak/ + , CD34weak/ + , CD19 + / + + , CD10 + + , CD38weak/ + , CD9 + + , CD20 + /variable, SMIg—with slightly higher side scatter than normal haematogones (Fig. 1).
The patient commenced imatinib 400 mg daily, achieving a major molecular response (MMR). Four years later, she progressed with abrupt lymphoblast crisis. The phenotype of the lymphoblasts was similar, but not identical, to those detected at diagnosis: CD45 + , CD34 + /partial, CD19 + / + + , CD10 + + , CD38 weak, CD9 weak, CD20 variable, HLA-DR + + , cCD79a + , CD11b weak, cTDT + , CD33 weak, SmIg-. She commenced treatment with dexamethasone, vincristine, and dasatinib, again achieving MMR.
Flow cytometry in CML chronic phase
A total of 65 patients with a new diagnosis of CML-CP were identified. Of these patients, 6 flow cytometry files were not available for review, and the final analysis was performed on the remaining 59 patients. The median age was 64 years (range 32–85 years) and 56% of patients were female. Fifty-seven patients had the p210 BCR-ABL1 fusion transcript; in one patient the p190 transcript was detected, and one did not have this information available. Two patients had BCR-ABL1 translocations involving additional chromosomes; one had a cryptic insertion detected on FISH and PCR but not on conventional karyotype. An additional 4 patients had abnormalities detected on conventional karyotype besides t(9;22); these were inv(2)(p25q21), t(5;15)(q13;q22), and two patients with -Y.
The patient reported above is one of four patients we identified with an abnormal lymphoblast population (Table 2). The index patient had the highest percentage of aberrant lymphoblasts and was the only patient reported prospectively with confidence. The immunophenotype in those with abnormal lymphoblast populations was similar but distinct from haematogones. The median percentage of haematogones in these patients was 0.36% (44% of B cells) compared to 0.05% (16% of B cells) in those without abnormal lymphoblasts. These patients otherwise had similar age, myeloblast percentage, and incidence of aberrancy to the remainder of the cohort. An additional four patients had abnormal mature B cell populations predominantly of a chronic lymphocytic leukaemia (CLL) phenotype (Table 3).
Unexpectedly, an abnormal myeloblast immunophenotype was detected in 88% of patients with all of these patients expressing weak CD11b (Table 3). Additional aberrant markers were detected in 34% with the most common being weak CD7.
Discussion
The 2017 revised 4th edition of the World Health Organisation classification of myeloid neoplasms and acute leukaemia includes abnormal lymphoblast populations in CML as a possible indicator of an aggressive disease course [5]. In our case study, the relationship between the aberrant lymphoblast population at diagnosis and the B-lymphoblastic leukaemia clone at transformation is interesting but has not been proven to be causative. Our pattern of referrals and follow-up after treatment did not permit detailed follow-up of most cases. However, in units where diagnostic and follow-up marrows tend to remain within one centre, it would be of interest to analyse diagnostic marrows in individuals treated for chronic phase CML who have subsequently transformed to B-lymphoblastic leukaemia with the objective of establishing whether there existed an aberrant precursor B-lymphoblast population in those individuals at diagnosis of chronic phase CML.
To date there have been 18 reported cases of abnormal lymphoblasts in CML-CP [6,7,8,9]. There is an overall high rate of transformation reported with 6/18 (33%) progressing to blast phase with follow-up of 1–67 months despite all patients receiving treatment with TKI therapy. The rates of progression vary significantly between studies, with five of the patients who progressed being included in two publications [8, 9]. This is in comparison to the publications by Vrotsos et al. and Soma et al. who reported only a single blast crisis from 11 patients [6, 7]. It is also important to note that of these 18 cases, at least six were children or adolescents, a group who may have a more aggressive clinical course than adults [10]. In those who did develop blast phase, the time to progression was not consistent between studies. In our clinical case, the patient had an abrupt progression with lymphoblast crisis several years following her diagnosis suggesting that if the risk of lymphoblast crisis is increased, it may not be imminent.
In many of the cases reported, as in our case, the lymphoblast populations were associated with a phenotype similar to haematogones. Common immunophenotypes included abnormally bright CD19, bright CD10, weaker CD38, and, in some cases, aberrant expression of CD20 on CD34 + cells [6, 7, 11]. In the cases described in this paper, haematogones comprised small percentages of cells overall. This is consistent with the typically marked granulocytic hyperplasia at an initial diagnosis of CML-CP. Comparing still these small percentages amongst our cases analysed, we noticed a trend towards marginally more prominent hematogones in the marrows of patients who had abnormal B-precursors identified relative to those marrows where CML-CP was diagnosed without abnormal B-precursors. We also noted, as previously documented, that in all cases where aberrant B-precursors were present in the marrow, there were at least equivalent percentages or greater of normal hematogones present [6, 7]. A thorough examination of the phenotype of these relatively small populations of B-cell precursors is required to identify and contrast the abnormal populations with the typical precursor B-cell phenotype, but the presence of normal precursors is useful in this regard.
The incidence of abnormal lymphoblast populations reported in these publications ranged from 2 to 11%, and in our cohort was 7%. As flow cytometry is often not performed in CML-CP it is possible that these populations may be underrecognised [9]. Alternatively, this high incidence may reflect reporting bias, with centers only publishing cases where they have detected aberrant populations, and particularly when these have been associated with subsequent development of blast phase.
It has been traditionally thought that CML-CP involves granulocytic proliferation without immunophenotypic aberrancy. We had the unexpected finding of aberrant myeloblasts in the majority of patients with a high incidence of CD11b expression on CD34 + myeloblasts and approximately one third expressing additional aberrant markers, most commonly CD7. It is noted that CD11b expression on myeloid blasts in CML-CP has in fact been described in the literature. Janssen et al. found CD11b expression on a proportion of stem cells which were Philadelphia chromosome positive, whereas normal stem cells were generally CD11b negative or low [12]. Our finding of CD11b expression on > 20% of myeloid blasts in 88% of our CML-CP cases is in keeping with this. We have reported CD7 expression on blasts as aberrant although the literature is not unified in whether weak CD7 may be expressed in normal early stem cell to myeloid differentiation [13]. Moreover CD7 + blasts were also CD11b + , increasing the level of confidence of aberrance.
Previous studies have noted the presence of aberrant myeloblasts co-expressing CD7 or CD56 in just over a quarter of CML-CP [9]. The implications of these aberrant myeloblasts requires further study with small case series reporting inferior outcomes in patients with aberrant stem cells including the presence of CD7, CD11b, and CD56 compared to those who did not display any aberrant markers [12].
The limitations of this study include its retrospective design; however, all flow cytometry was prospectively re-analysed and blinded from previously reported results and patient outcomes. Additionally, several cases were identified where an abnormal population may have been present however due to low numbers of events, below our defined threshold, these were not reported. Finally, complete follow-up could not be guaranteed for our cases identified (besides that reported in the index case) and as such has not been included.
This case highlights the ongoing possibility of a relationship between abnormal lymphoblast populations at diagnosis in CML and the risk of blast crisis which continues in the era of TKI therapy. More studies preferably with prospective rare event analyses are required to further define the frequency of abnormal lymphoblast populations in CML and the risk of progression in these patients.
References
Hochhaus A, Larson RA, Guilhot F et al (2017) Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med 376(10):917–927. https://doi.org/10.1056/NEJMoa1609324
Jain P, Kantarjian HM, Ghorab A et al (2017) Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: Cohort study of 477 patients. Cancer 123(22):4391–4402. https://doi.org/10.1002/cncr.30864
Hasford J, Baccarani M, Hoffmann V et al (2011) Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood 118(3):686–692. https://doi.org/10.1182/blood-2010-12-319038
Sokal JE, Cox EB, Baccarani M et al (1984) Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood 63(4):789–799
Arber DA, Orazi A, Hasserjian R et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127(20):2391–2405. https://doi.org/10.1182/blood-2016-03-643544
Vrotsos E, Gorgan M, DiGiuseppe JA (2017) Detection of small abnormal B-Lymphoblast populations at diagnosis of chronic myelogenous leukemia, BCR-ABL1+: Incidence, phenotypic features, and clinical implications. Cytometry B Clin Cytom 92(4):275–278. https://doi.org/10.1002/cyto.b.21250
Soma L, Oehler VG, Ding C, Cherian S (2016) Small, abnormal B lymphoid blast populations in chronic myelogenous leukemia at diagnosis: Does this finding indicate an accelerated course? Cytometry B Clin Cytom 90(5):440–448. https://doi.org/10.1002/cyto.b.21357
Vijayasekharan K, Chatterjee G, Ramanathan S et al (2020) Sudden blast phase in pediatric chronic myeloid leukemia-chronic phase with abnormal lymphoid blasts detected by flow cytometry at diagnosis: can it be considered a warning sign? Cytometry B Clin Cytom. https://doi.org/10.1002/cyto.b.21958
El Rassi F, Bergsagel JD, Arellano M et al (2015) Predicting early blast transformation in chronic-phase chronic myeloid leukemia: is immunophenotyping the missing link? Cancer 121(6):872–875. https://doi.org/10.1002/cncr.29142
Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M (2016) Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood 127(4):392–399. https://doi.org/10.1182/blood-2015-06-648667
McKenna RW, Washington LT, Aquino DB, Picker LJ, Kroft SH (2001) Immunophenotypic analysis of hematogones (B-lymphocyte precursors) in 662 consecutive bone marrow specimens by 4-color flow cytometry. Blood 98(8):2498–2507. https://doi.org/10.1182/blood.v98.8.2498
Janssen JJ, Deenik W, Smolders KG et al (2012) Residual normal stem cells can be detected in newly diagnosed chronic myeloid leukemia patients by a new flow cytometric approach and predict for optimal response to imatinib. Leukemia 26(5):977–984. https://doi.org/10.1038/leu.2011.347
Kriegsmann K, Loffler H, Eckstein V et al (2018) CD7 is expressed on a subset of normal CD34-positive myeloid precursors. Eur J Haematol 101(3):318–325. https://doi.org/10.1111/ejh.13100
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Luani Barge: data curation, formal analysis, writing—original draft. Rebecca Cleary: writing—review and editing. Kirk Morris: writing—review and editing. Erin Simleit: conceptualisation, formal analysis, supervision, writing—review and editing. Rebecca Cleary and Kirk Morris were involved in the care of the patient presented.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Barge, L., Cleary, R., Morris, K. et al. Incidence and immunophenotype of abnormal lymphoblast populations at diagnosis of chronic myeloid leukaemia in chronic phase. J Hematopathol 15, 51–56 (2022). https://doi.org/10.1007/s12308-022-00487-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12308-022-00487-7