Abstract
The insect pests are big threat in meeting the food demands for future generation. The present pest control strategies, including the existing transgenic approaches show certain limitations and are not completely successful in limiting the insect pests. However, the sequence-specific gene silencing via RNA interference (RNAi) holds a great promise for effective management of agricultural pests. RNAi is naturally occurring conserved process responsible for gene regulation and defense against pathogens. The efficacy of RNAi varies among different insect orders and also depends upon various factors, including the target gene selection, method of dsRNAs delivery, expression of dsRNAs and presence of off-target effects. RNAi-mediated silencing of different insect genes involved in various physiological processes was found to be detrimental to insects growth, development and survival. In this article, we have reviewed the potential of RNAi-based strategies for effective management of insect pests. We have also discussed the various parameters, which are to be considered for host-induced RNAi-mediated control of insect pests without producing any effect on non-target organisms and environment.

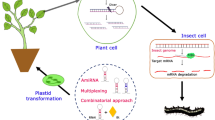
(Source adopted from Palli 2014)

Similar content being viewed by others
References
Allen ML, Walker WB (2012) Saliva of Lygus lineolaris digests double stranded ribonucleic acids. J Insect Physiol 58:391–396
Andrade CE, Hunter WB (2016) RNA interference—natural gene-based technology for highly specific pest control (HiSPeC). In: Abdurakhmonov IY (ed) RNA interference. InTech, Croatia, pp 391–409
Araujo RN, Santos A, Pinto FS, Gontijo NF, Lehane MJ, Pereira MH (2006) RNA interference of the salivary gland nitrophorin 2 in the triatomine bug Rhodnius prolixus (Hemiptera: Reduviidae) by dsRNA ingestion or injection. Insect Biochem Mol Biol 36:683–693
Attardo GM, Benoit JB, Michalkova V, Yang G, Roller L, Bohova J, Takácˇ P, Aksoy S (2012) Analysis of lipolysis underlying lactation in the tsetse fly, Glossina morsitans. Insect Biochem Mol Biol 42:360–370
Bardner R, Fletcher KE (1974) Insect infestations and their effects on the growth and yield of field crops: a review. Bull Entomol Res 64:141–160
Batzri S, Korn ED (1973) Single bilayer liposomes prepared without sonication. Biochim Biophys Acta 16:1015–1019
Baum JA, Roberts JK (2014) Progress towards RNAi-mediated insect pest management. Adv Insect Physiol 47:249–295
Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P et al (2007) Control of coleopteran insect pests through RNA interference. Nat Biotechnol 25:1322–1326
Belles X (2010) Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu Rev Entomol 55:111–128
Bhatia V, Bhattacharya R, Uniyal PL, Singh R, Niranjan RS (2012) Host generated siRNAs attenuate expression of serine protease gene in Myzus persicae. PLoS One 7:e46343
Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D et al (2006) 3′ UTR seed matches, but not overall identities, are associated with RNAi off-targets. Nat Methods 3:199–204
Boisson B, Jacques JC, Choumet V, Martin E, Xu J, Vernick K, Bourgouin C (2006) Gene silencing in mosquito salivary glands by RNAi. FEBS Lett 580:1988–1992
Bolognesi R, Ramaseshadri P, Anderson J, Bachman P, Clinton W et al (2012) Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 7:e47534
Bucher G, Scholten J, Klingler M (2002) Parental RNAi in Tribolium (Coleoptera). Curr Biol 12:85–86
Burand JP, Hunter WB (2013) RNAi: future in insect management. J Invertebr Pathol 112:S68–S74
Cappelle K, de Oliveira CF, Van Eynde B, Christiaens O, Smagghe G (2016) The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in double-stranded RNA uptake in the Colorado potato beetle midgut. Insect Mol Biol 25:315–323
Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136:642–655
Christou P, Capell T, Kohli A, Gatehouse JA, Gatehouse AMR (2006) Recent developments and future prospects in insect pest control in transgenic crops. Trends Plant Sci 11:302–308
Cogoni C, Irelan JT, Schumacher M, Schmidhauser T, Selker EU, Macino G (1996) Transgene silencing of the al-1 gene in vegetative cells of Neurosporais mediated by a cytoplasmic effectors and does not depend on DNA–DNA interactions or DNA methylation. EMBO J 15:3153–3163
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Ganbaatar O, Cao B, Zhang Y, Bao D, Bao W, Wuriyanghan H (2017) Knockdown of Mythimna separata chitinase genes via bacterial expression and oral delivery of RNAi effectors. BMC Biotechnol 17:9
Garbutt JS, Belles X, Richards EH, Reynolds SE (2013) Persistence of double-stranded RNA in insect hemolymph as a potential determiner of RNA interference success: evidence from Manduca sexta and Blattella germanica. J Insect Physiol 59:171–178
Gordon KH, Waterhouse PM (2007) RNAi for insect-proof plants. Nat Biotechnol 25:1231–1232
Gu L, Knipple DC (2013) Recent advances in RNA interference research in insects: implications for future insect pest management strategies. Crop Prot 45:36–40
Hakim RS, Baldwin K, Smagghe G (2010) Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol 55:593–608
Hilder VA, Boulter D (1999) Genetic engineering of crop plants for insect resistance—a critical review. Crop Prot 18:177–191
Howard KA, Rahbek UL, Liu X, Damgaard CK, Glud SZ, Andersen MØ, Hovgaard MB, Schmitz A, Nyengaard JR, Besenbacher F, Kjems J (2006) RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol Ther 14:476–484
Huang J, Pray C, Rozella S (2002) Enhancing the crops to feed the poor. Nature 418:678–683
Huvenne H, Smagghe G (2010) Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 56:227–235
James C (2014) Global status of commercialized Biotech/GM crops. ISAAA Brief 49. ISAAA, Ithaca, NY
Jin S, Singh ND, Li L, Zhang X, Daniell H (2015) Engineered chloroplast dsRNA silences cytochrome p450 monooxygenase, V-ATPase and chitin synthase genes in the insect gut and disrupts Helicoverpa armigera larval development and pupation. Plant Biotech J 13:435–446
Joga MR, Zotti MJ, Smagghe G, Christiaens O (2016) RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: what we know so far. Front Physiol 7:553
Jonathan GL, Jian JD (2013) RNAi-based insecticidal crops: potential effects on nontarget species. Bioscience 63:657–666
Jose AM, Hunter CP (2007) Transport of sequence-specific RNA interference information between cells. Annu Rev Genet 41:305–330
Jose AM, Smith JJ, Hunter CP (2009) Export of RNA silencing from C. elegans tissues does not require the RNA channel SID-1. Proc Natl Acad Sci USA 106:2283–2288
Kitzmann P, Schwirz J, Schmitt-Engel C, Bucher G (2013) RNAi phenotypes are influenced by the genetic background of the injected strain. BMC Genom 14:5
Knip M, Constantin ME, Thordal-Christensen H (2014) Trans-kingdom cross-talk: small RNAs on the move. PLoS Genet 10:e1004602
Koch A, Biedenkopf D, Furch A, Weber L, Rossbach O et al (2016) An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 12:e1005901
Kola VSR, Renuka P, Madhav MS, Mangrauthia SK (2015) Key enzymes and proteins of crop insects as candidate for RNAi based gene silencing. Front Physiol 6:119
Konakalla NC, Kaldis A, Berbati M, Masarapu H, Voloudakis AE (2016) Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta 24:961–969
Kumar M, Gupta GP, Rajam MV (2009) Silencing of acetylcholinesterase gene of Helicoverpa armigera by siRNA affects larval growth and its life cycle. J Insect Physiol 55:273–278
Kumar P, Pandit SS, Baldwin IT (2012) Tobacco rattle virus vector: a rapid and transient means of silencing Manduca sexta genes by plant mediated RNA interference. PLoS One 7:e31347
Lal OP (1985) Field resistance of some tomato cultivars against the fruitworm, Heliothis armigera (Hubner). Tripoli. Bull Ent 26:46–47
Li H, Chougule NP, Bonning BC (2011) Interaction of the Bacillus thuringiensis delta endotoxins Cry1Ac and Cry3Aa with the gut of the pea aphid, Acyrthosiphon pisum (Harris). J Invertebr Pathol 107:69–78
Li H, Jiang W, Zhang Z, Xing Y, Li F (2013) Transcriptome analysis and screening for potential target genes for RNAi-mediated pest control of the beet armyworm, Spodoptera exigua. PLoS One 8:e65931
Li H, Guan R, Guo H, Miao X (2015) New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ 38:2277–2285
Lipardi C, Paterson BM (2009) Identification of an RNA-dependent RNA polymerase in Drosophila involved in RNAi and transposon suppression. Proc Natl Acad Sci USA 106:15645–15650
Liu S, Ding Z, Zhang C, Yang B, Liu Z (2010) Gene knockdown by introthoracic injection of double-stranded RNA in the brown planthoer, Nilaparvata lugens. Insect Biochem Mol Biol 40:666–667
Liu J, Smagghe G, Swevers L (2013) Transcriptional response of BmToll9-1 and RNAi machinery genes to exogenous dsRNA in the midgut of Bombyx mori. J Insect Physiol 59:646–665
Liu F, Wang XD, Zhao YY, Li YJ, Liu YC, Sun J (2015) Silencing the HaAK gene by transgenic plant-mediated RNAi impairs larval growth of Helicoverpa armigera. Int J Biol Sci 11:67–74
Lomazzo E, Hussmann GP, Wolfe BB, Yasuda RP, Perry DC, Kellar KJ (2011) Effects of chronic nicotine on heteromeric neuronal nicotinic receptors in rat primary cultured neurons. J Neurochem 119:153–164
Malik HJ, Raza A, Amin I, Scheffler JA, Scheffler BE, Brown JK, Mansoor S (2016) RNAi-mediated mortality of the whitefly through transgenic expression of double-stranded RNA homologous to acetylcholinesterase and ecdysone receptor in tobacco plants. Sci Rep 6:38469
Mamta, Reddy KRK, Rajam MV (2016) Targeting chitinase gene of Helicoverpa armigera by host-induced RNA interference confers insect resistance in tobacco and tomato. Plant Mol Biol 90:281–292
Mao J, Zeng F (2014) Plant-mediated RNAi of a gap gene-enhanced tobacco tolerance against the Myzus persicae. Transgenic Res 23:145–152
Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, Huang YP, Chen X (2007) Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol 25:1307–1313
Mao YB, Tao XY, Xue XY, Wang LJ, Chen XY (2011) Cotton plants expressing CYP6AE14 double-stranded RNA show enhanced resistance to bollworms. Transgenic Res 20:665–673
Mao YB, Xue XY, Tao XY, Yang CQ, Wang LJ, Chen XY (2013) Cysteine protease enhances plant-mediated bollworm RNA interference. Plant Mol Biol 83:119–129
Mehto DN, Singh KM, Singh RN (1985) Incidence of insect pests in chickpea, Cicer arietinum L. Indian J Ent 47:117–136
Miguel SK, Scott JG (2016) The next generation of insecticides: dsRNA is stable as a foliar-applied insecticide. Pest Manag Sci 72:801–809
Miller SC, Brown SJ, Tomoyasu Y (2008) Larval RNAi in Drosophila? Dev Genes Evol 218:505–510
Miller SC, Miyata K, Brown SJ, Tomoyasu Y (2012) Dissecting systemic RNA interference in the red flour beetle Tribolium castaneum: parameters affecting the efficiency of RNAi. PLoS ONE 7:e47431
Napoli C, Lemieux C, Jorgensen R (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous gene in trans. Plant Cell 2:279–289
Navale PM, Maligeppagol M, Mahadeva Swamy HM, Ramasamy A, Sowmya HD, Krishna V, Prasad Babu K, Kumbar BM (2014) Plant mediated RNAi: a new line of defense against insect pests. Int J Biotechnol Appl 6:163–168
Nellen W, Lichtenstein C (1993) What makes an mRNA antisenseitive? Trends Biol Sci 18:419–423
Oerke EC (2006) Crop losses to pests. J Agric Sci 144:31–43
Orii H, Mochii M, Watanabe K (2003) A simple ‘‘soaking method’’ for RNA interference in the planarian Dugesia japonica. Dev Genes Evol 213:138–141
Palli SR (2014) RNA interference in Colorado potato beetle: steps toward development of dsRNA as a commercial insecticide. Curr Opin Insect Sci 6:1–8
Perrimon N, Ni JQ, Perkins L (2010) In vivo RNAi: today and tomorrow. Cold Spring Harb Perspect Biol 2:a003640
Pitino M, Coleman AD, Maffei ME, Ridout CJ, Hogenhout SA (2011) Silencing of aphid genes by dsRNA feeding from plants. PLoS One 6:e25709
Prentice K, Pertry I, Christiaens O, Bauters L, Bailey A, Niblett C, Ghislain M, Gheysen G, Smagghe G (2015) Transcriptome analysis and systemic RNAi response in the African sweet potato weevil (Cylas puncticollis, Coleoptera, Brentidae). PLoS ONE 10:e0115336
Price DR, Gatehouse JA (2008) RNAi-mediated crop protection against insects. Trends Biotechnol 26:393–400
Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R et al (2008) The genome of the model beetle and pest Tribolium castaneum. Nature 452:949–955
Rinkevich FD, Scott JG (2013) Limitations of RNAi of α6 nicotinic acetylcholine receptor subunits for assessing the in vivo sensitivity to spinosad. Insect Sci 20:101–108
Romano N, Macino G (1992) Quelling: transient inactivation of gene expression in Neurospora crassa by transformation with homologous sequences. Mol Microbiol 6:3343–3353
Roush DK, McKenzie JA (1987) Ecological genetics of insecticide and acaricide resistance. Annu Rev Entomol 32:361–380
Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O’Farrell PH, Andino R (2006) The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol 8:793–802
Saurabh S, Vidyarthi AS, Prasad D (2014) RNA interference: concept to reality in crop improvement. Planta 239:543–564
Shakesby AJ, Wallace IS, Isaacs HV, Pritchard J, Roberts DM, Douglas AE (2009) A water-specific aquaporin involved in aphid osmoregulation. Insect Biochem Mol Biol 39:1–10
Sharma DK, Sharma T (2013) Biotechnological approaches for biodiversity conservation. Indian J Sci Res 4:183–186
Sharma HC, Crouch JH, Sharma KK, Seetharama N, Hash CT (2002) Applications of biotechnology for crop improvement: prospects and constraints. Plant Sci 63:381–395
Shukla JN, Kalsi M, Sethi A, Narva KE, Fishilevich E, Singh S, Mogilicherla K, Palli SR (2016) Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol 13:656–669
Stenersen J (2004) Chemical pesticides: modes of action and toxicology. CRC Press, Boca Raton
Stevens J, Dunse K, Fox J, Evans S, Anderson M (2012) Biotechnological approaches for the control of insect pests in crop plants. In: Soundararajan RP (ed) Pesticides-advances in chemical and botanical pesticides. InTech, Croatia. doi:10.5772/46233
Tabara H, Grishok A, Mello CC (1998) RNAi in C. elegans soaking in the genome sequence. Science 282:430–431
Tabashnik BE, Brévault T, Carrière Y (2013) Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31:510–521
Taning CNT, Christiaens O, Berkvens N, Casteels H, Maes M, Smagghe G (2016) Oral RNAi to control Drosophila suzukii: laboratory testing against larval and adult stages. J Pest Sci 89:803–814
Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H et al (2011) RNA interference in lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol 57:231–245
Thakur N, Upadhyay SK, Verma PC, Chandrashekar K, Tuli R, Singh PK (2014) Enhanced whitefly resistance in transgenic tobacco plants expressing double stranded RNA of v-ATPase A gene. PLoS ONE 9:e87235
Thakur N, Mundey JK, Upadhyay SK (2016) RNAi-implications in entomological research and pest control. In: Abdurakhmonov IY (ed) RNA interference. InTech, Croatia. doi:10.5772/61814
Tian H, Peng H, Yao Q, Chen H, Xie Q, Tang B, Zhang W (2009) Developmental control of a lepidopteran pest Spodoptera exigua by ingestion of bacteria expressing dsRNA of a non-midgut gene. PLoS ONE 4:e6225
Timmons L, Fire A (1998) Specific interference by ingested dsRNA. Nature 395:85
Tomoyasu Y, Miller SC, Shuichiro T, Schomeier M, Grossmann D, Bucher G (2008) Exploring systemic RNA interference in insects: a genome-wide survey for RNAi genes in Tribolium. Genome Biol. doi:10.1186/gb-2008-9-1-r10
Ulrich J, Dao VA, Majumdar U, Schmitt-Engel C, Schwirz J et al (2015) Large scale RNAi screen in Tribolium reveals novel target genes for pest control and the proteasome as prime target. BMC Genom 16:674
Ulvila J, Parikka M, Kleino A, Sormunen R, Ezekowitz RA, Kocks C, Rämet M (2006) Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J Biol Chem 281:14370–14375
Vinokurov KS, Elpidina EN, Oppert B, Prabhakar S, Zhuzhikov DP, Dunaevsky YE, Belozersky MA (2006) Diversity of digestive proteinases in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Comp Biochem Physiol 145:126–137
Walshe DP, Lehane SM, Lehane MJ, Haines LR (2009) Prolonged gene knockdown in the tsetse fly Glossina by feeding double stranded RNA. Insect Mol Biol 18:11–19
Whyard S, Singh AD, Wong S (2009) Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem Mol Biol 39:824–832
Wilson RC, Doudna JA (2013) Molecular mechanisms of RNA interference. Annu Rev Biophys 42:217–239
Winston WM, Molodowitch C, Hunter CP (2002) Systemic RNAi in C. elegans requires the putative transmembrane protein SID-1. Science 295:2456–2459
Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G et al (2007) Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol 25:1149–1157
Wynant N, Santos D, Verdonck R, Spit J, Van Wielendaele P, Vanden Broeck J (2014) Identification, functional characterization and phylogenetic analysis of double stranded RNA degrading enzymes present in the gut of the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol 46:1–8
Xiong Y, Zeng H, Zhang Y, Xu D, Qiu D (2013) Silencing the HaHR3 gene by transgenic plant-mediated RNAi to disrupt Helicoverpa armigera development. Int J Biol Sci 9:370–381
Xu J, Wang XF, Chen P, Liu FT, Zheng SC, Ye H, Mo MH (2016) RNA interference in moths: mechanisms, applications, and progress. Genes 7:88
Xue XY, Mao YB, Tao XY, Huang YP, Chen XY (2012) New approaches to agricultural insect pest control based on RNA interference. Adv Insect Physiol 42:73–117
Yencho GC, Cohen MB, Byrne PF (2000) Applications of tagging and mapping insect resistance loci in plants. Annu Rev Entomol 45:393–422
Yogindran S, Rajam MV (2015) RNAi for crop improvement. In: Bahadur Bir et al (eds) Plant biotechnology: volume II: plant genomics and biotechnology. Springer, New Delhi, pp 623–637
Yu R, Xu X, Liang Y, Tian H, Pan Z, Jin S, Wang N, Zhang W (2014) The insect ecdysone receptor is a good potential target for RNAi-based pest control. Int J Biol Sci 10:1171–1180
Yu N, Christiaens O, Liu J, Niu J, Caelle K, Caccia S, Huvenne H, Smagghe G (2013) Delivery of dsRNA for RNAi in insects: an overview and future directions. Insect Sci 20:4–14
Zha WJ, Peng XX, Chen RZ, Du B, Zhu LL, He GC (2011) Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the Hemipteran insect Nilaparvata lugens. Plos One 6:e20504
Zhang X, Zhang J, Zhu KY (2010) Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol Biol 19:683–693
Zhang H, Li HC, Miao XX (2013) Feasibility, limitation and possible solutions of RNAi-based technology for insect pest control. Insect Sci 20:15–30
Zhu JQ, Liu S, Ma Y, Zhang JQ, Qi HS, Wei ZJ, Yao Q, Zhang WQ, Li S (2012) Improvement of pest resistance in transgenic tobacco plants expressing dsRNA of an insect associated gene EcR. PLoS ONE 7:e38572
Zotti MJ, Smagghe G (2015) RNAi technology for insect management and protection of beneficial insects from diseases: lessons, challenges and risk assessments. Neotrop Entomol 44:197–213
Acknowledgements
We are grateful to the Department of Biotechnology (Grant No. BT/AGR/TF/2006), New Delhi for generous support for RNAi work in the lab (to MVR). Mamta acknowledges the University Grants Commission, New Delhi for the senior research fellowship under the special assistance programme (SAP) of University Grants Commission (UGC). We also thank the UGC for SAP (DRS-III), Department of Science and Technology (DST), New Delhi for FIST (Level 2) programme and DU-DST PURSE (Phase II) Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have conflict of interests.
Rights and permissions
About this article
Cite this article
Mamta, B., Rajam, M.V. RNAi technology: a new platform for crop pest control. Physiol Mol Biol Plants 23, 487–501 (2017). https://doi.org/10.1007/s12298-017-0443-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-017-0443-x