Abstract
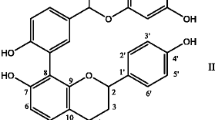
The main objective of this study is to evaluate the anti-hypertrophic potential of the aqueous extract of Enicostemma littorale (E. littorale) against isoproterenol induced cardiac hypertrophic rat models (male albino Wistar rats) through biochemical investigations. Aqueous extract of E. littorale known for various beneficial properties was administered (100 mg/kg, 12 days, oral) to isoproterenol (ISO) induced cardiac hypertrophic rats (low ISO—60 mg/kg, 12 days and high ISO—100 mg/kg, 12 days, subcutaneous) and were compared with group that was treated with the reference drug, Losartan (10 mg kg, administered for 12 days, oral). The anti-hypertrophic effect of E. littorale was evaluated by analysing the morphometric indices of the heart, ECG tracings, changes in blood biochemical parameters viz., serum glucose, serum total protein, serum albumin, lipid profile, cardiac specific enzymes (SGOT, SGPT and LDH) and histopathological examination of the heart tissue. The results fundamentally revealed that the plant extract efficiently ameliorated cardiac hypertrophy induced by ISO injected in experimental rats. The outcomes of biochemical investigations of this study highlighted the association between the hypertrophic β-adrenergic receptor signalling (β-AR) and the 5′ AMP-activated protein kinase (AMPK)—peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) axis in the metabolism of cardiac fibrosis and hypertrophy. This β-AR/AMPK-PGC1α signalling stem can serve as a key target in ameliorating cardiac hypertrophy through focus on its principal regulators. To add, we also propose that the glycoside, swertiamarin present in this plant with the reported anti-fibrotic potential in liver can be further isolated and evaluated for its anti-hypertrophic potential to treat cardiac hypertrophy.





Similar content being viewed by others
References
Al Makdessi S, Andrieu JL, Herilier H, Faucon G. Effect of isoproterenol on the metabolism of myocardial fatty acids. J Mol Cell Cardiol. 1987;19:141–9.
Al-Rasheed NM, Al-Oteibi MM, Al-Manee RZ, Al-Shareef NM, Hasan IH, Mohamad RA, Mahmoud AM. Simvastatin prevents isoproterenol-induced cardiac hypertrophy through modulation of the JAK/STAT pathway. Drug Des Dev Ther. 2015;9:3217–29.
Beauloye C, Bertrand L, Horman S, Hue L. AMPK activation, a preventive therapeutic target in the transition from cardiac injury to heart failure. Cardiovasc Res. 2011;90:224–33.
Brooks WW, Conrad CH. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: structural and functional correlates. Comp Med. 2009;59(4):339–43.
Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105.
Chang GR, Chen WK, Hou PH, Mao FC. Isoproterenol exacerbates hyperglycaemia and modulates chromium distribution in mice fed with a high fat diet. J Trace Elem Med Biol. 2017;44:315–21.
Chen K, Wu T, Zhang R, Song H. Effects of Swertiamarin on TGF-β1/Smad signalling pathway in rats with carbon tetrachloride-induced liver fibrosis. Int J Clin Exp Med. 2017;10(2):2316–25.
Chikkamath V, Manjusha MP, Shanmukha I. The effect of Enicostemma littorale Blume on adrenaline-induced hypertensive rats. J Pharm Res. 2017;16(3):224–8.
Choudhary R, Mishra KP, Subramanyam C. Prevention of isoproterenol induced cardiac hypertrophy by Eugenol, an antioxidant. Indian J Clin Biochem. 2006;21(2):107–13.
Chowdhury D, Tangutur AD, Khatua TN, Saxena P, Banerjee SK, Bhadra MP. A proteomic view of isoproterenol induced cardiac hypertrophy: Prohibitin identified as a potential biomarker in rats. J Transl Med. 2013;11:130–43.
Deshaies Y, LeBlanc J, Willemot J. Studies on protein metabolism during isoproterenol-induced cardiac hypertrophy. Recent Adv Stud Card Struct Metab. 1975;8:387–95.
Doss VA, Kuberapandian D. Antidepressant activity of Enicostemma littorale Blume in Shp2 (Protein tyrosine phosphatase)-inhibited animal model of depression. Int J Prev Med. 2016;7:112.
Goyal KR, Patel N, Khanna V. Swertiamarin: possible answer for diabetic cardiomyopathy. Abstr Curr Res Cardiol. 2014;1(1):43.
Handa SS, Khanuja SPS, Longo G, Rakesh DD. Extraction technologies for medicinal and aromatic plants. ICS UNIDO. 2008.
Jaehnig EJ, Heidt AB, Greene SB, Cornelissen I, Black BL. Increased susceptibility to Isoproterenol-induced cardiac hypertrophy and impaired weight gain in mice lacking the histidine-rich calcium-binding protein. Mol Cell Biol. 2006;26(24):9315–26.
Jemai H, Sayadi S. Heart histopathology and oxidative features in diabetic rats and protective effects of Oleuropein. Adv Biosci Biotechnol. 2015;6:383–9.
Leong XY, Thanikachalam PV, Pandey M, Ramamurthy S. A systematic review of the protective role of swertiamarin in cardiac and metabolic diseases. Biomed Pharmacother. 2016;84:1051–60.
Li S, Wang Q, Tao Y, Liu C. Swertiamarin attenuates experimental rat hepatic fibrosis by suppressing Angiotensin II–Angiotensin type 1 receptor-extracellular signal-regulated kinase signalling. J Pharmcol Exp Ther. 2016;359:247–55.
Lobo RO, Shenoy CK. Myocardial potency of bio-tea against isoproterenol induced myocardial damage in rats. J Food Sci Technol. 2015;52(7):4491–8.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Manoharan S, Rajasekaran D, Prabhakar MM, Karthikeyan S, Manimaran A. Modulating effect of Enicostemma littorale on the expression pattern of apoptotic, cell proliferative, inflammatory and angiogenic markers during 7,12-dimethylbenz(a)anthracene induced Hamster buccal pouch carcinogenesis. Toxicol Int. 2015;22(1):130–40.
Mesquita TRR, de Jesus ICG, dos Santos JF, de Almeida GKM, de Vasconcelos CML, Guatimosism S, Macedo FN, dos Santos RV, de Menezes-Filho JER, dos Santos RM, Matos PTD, Scalzo S, Filho VJS, Junior RLCA, Filho RNP, Santos SL. Cardioprotective action of Ginkgo biloba extract against sustained β-adrenergic stimulation occurs via activation of M2/NO pathway. Front Pharmacol. 2017;8(220):1–13.
Moon KH, Song IS, Yang WS, Shin YT, Kim SB, Song JK, Park JS. Hypoalbuminemia as a risk factor for progressive left-ventricular hypertrophy in haemodialysis patients. AMJ Nephrol. 2000;20(5):396–401.
Patel TP, Soni S, Parikh O, Gosai J, Chruvattil, Gupta S. Swertiamarin: an active lead from Enicostemma littorale regulates hepatic and adipose tissue gene expression by targeting PPAR-γ and improves insulin sensitivity in experimental NIDDM rat model. Evid Based Complement Altern Med. 2013; 1–11.
Planavila A, Redondo I, Hindares E, Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt M, Bilsen MV, Villarroya F. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat Commun. 2013;4:1–12.
Roy SP, Niranjan CM, Jyothi TM, Shankrayya MM, Vishawanath KM, Prabhu K, Gouda VA, Setty RS. Antiulcer and anti-inflammatory activity of aerial parts Enicostemma littorale Blume. J Young Pharm. 2010;2(4):369–73.
Sager G, Bratlid H, Little C. Binding of catecholamines to alpha-1 acid glycoprotein, albumin and lipoproteins in human serum. Biochem Pharmacol. 1987;36(21):3607–12.
Saranya R, Thirumalai T, Hemalatha M, Ranganathan B, David E. Pharmacognosy of Enicostemma littorale: a review. Asian Pac J Trop Biomed. 2013;3(1):79–84.
Shah PS, Sorenson LL, Abraham MR, Gabrielson KL. Electrocardiographic characterization of cardiac hypertrophy in mice that overexpresses the ErbB2 receptor tyrosine kinase. Comp Med. 2015;65(4):295–307.
Silva VJD, Neto EF, Salgadzo HC, Junior RF. Chronic converting enzyme inhibition normalizes QT interval in ageing rats. Braz J Med Biol Res. 2002;35:1025–31.
Snedecor GW, Cochran WG. Shortcut and non-parametric methods. In: Statistical methods; 1986.
Sonawane DR, Vishwakarma LS, Lakshmi S, Rajani M, Padh H, Goyal KR. Amelioration of STZ-induced type-1 diabetic nephropathy by aqueous extract of Enicostemma littorale Blume and swertiamarin in rats. Mol Cell Biochem. 2010;340:1–6.
Sozio MS, Lu C, Zeng Y, Liangpunsakul S, Crabb DW. Activated AMPK inhibits PPAR-α and PPAR-γ transcriptional activity in hepatoma cells. Am J Physiol Gastrointest Liver Physiol. 2011;301(4):G739–47.
Summermatter S, Santos G, Schindler JP, Handschin C. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. PNAS. 2013;10(21):8738–43.
Tavares P, Res F, Ribeiro CAF, Teixeira F. Cardiovascular effects of cyclosporin treatment in an experimental model. Rev Port Cardiol. 2002;21(2):141–55.
Waghabi MC, de Souza EM, de Oliveira GM, Keramidas M, Feige JJ, Araujo-Jorge TC, Bailly S. Pharmacological inhibition of transforming growth factor β signalling decreases infection and prevents heart damage in acute Chagas disease. Antimicrob Agents Chemother. 2009;53(11):4694–701.
Wang R, Wang Y, Lin WK, Zhang Y, Liu W, Huang K, Terrar DA, Solaro RJ, Wang X, Ke Y, Lei M. Inhibition of angiotensin II-induced cardiac hypertrophy and associated ventricular arrhythmias by a p21 activated kinase 1 bioactive peptide. PLoS ONE. 2014;9(7):1–12.
Wu T, Li J, Li Y, Song H. Antioxidant and hepatoprotective effect of swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cell Physiol Biochem. 2017;41:2242–54.
Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A, Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–8.
Zhang J, Knapton A, Lipshultz SE, Weaver JL, Herman EH. Isoproterenol-induced cardiotoxicity in Sprague-Dawley rats—correlation of reversible and irreversible myocardial injury with release of cardiac troponin T and roles of iNOS in myocardial injury. Toxicol Pathol. 2008;36:277–88.
Acknowledgements
We acknowledge the Botanical Survey of India, for providing the plant authentication. Thanks to PSG Institute of Medical Sciences and Research and PSG Institutional Animal Ethics Committee, Coimbatore, India and the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India for providing animal ethical clearance. We express gratitude for the services rendered by Ponmani & Co Pvt. Ltd., Coimbatore for the supply of Isoproterenol (Sigma) and Ratthan Labs, Coimbatore for the speedy preparation and examination of histopathological slides are appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
This article does not contain any studies involving human participants performed by any of the authors. The study was approved by the PSG Institutional Animal Ethics Committee (PSG Institute of Medical Sciences and Research), Coimbatore, India and the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India for providing animal ethical clearance (CPCSEA/No: 386/2018/IAEC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doss, V.A., Kuberapandian, D. Evaluation of Anti-hypertrophic Potential of Enicostemma littorale Blume on Isoproterenol Induced Cardiac Hypertrophy. Ind J Clin Biochem 36, 33–42 (2021). https://doi.org/10.1007/s12291-019-0814-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-019-0814-x