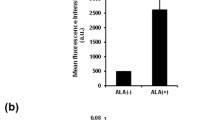
Abstract
Herein we develop a unique differentiated-uptake strategy capable of efficient and high-purity isolation of genuine drug-resistant (DR) cells from three types of drug-surviving cancer cells, which include paclitaxel-surviving human ovarian OVCAR-3 cancer cells and human lung carcinoma A549/Taxol cells, and doxorubicin-surviving human immortalized myelogenous leukemia K562/ADR cells. By using this strategy which relies on fluorescent glycan nanoparticle (FGNP)-based fluorescence-activated cell sorting (FACS) assays, two subpopulations with distinct fluorescences existing in drug-surviving OVCAR-3 cells were separated, and we found that the lower fluorescence (LF) subpopulation consisted of DR cells, while the higher fluorescence (HF) subpopulation was comprised of non-DR cells. Besides, the DR cells and their progenies were found distinct in their increased expression of drug-resistant genes. More intriguingly, by using the FGNP-based FACS assay to detect DR/non-DR phenotypes, we found that the DR phenotype had a potential to differentiate into the non-DR progeny, which demonstrates the differentiation feature of stem-like cancer cells. Further research disclosed that the assay can quantitatively detect the degree of drug resistance in DR cells, as well as the reversal of drug resistance that are tackled by various therapeutic methods. The strategy thus paves the way to develop theranostic approaches associated with chemotherapy-resistance and cancer stemness.

Similar content being viewed by others
Change history
24 June 2021
An Erratum to this paper has been published: https://doi.org/10.1007/s12274-021-3672-9
References
Holohan, C.; Van Schaeybroeck, S.; Longley, D. B.; Johnston, P. G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726.
Donnenberg, V. S.; Donnenberg, A. D. Multiple drug resistance in cancer revisited: The cancer stem cell hypothesis. J. Clin. Pharmacol. 2005, 45, 872–877.
Wang, C. L.; Wang, F. C.; Zhang, J. G.; Liu, L. S.; Xu, G. X.; Dou, H. J. Fluorescent polysaccharide nanogels for the detection of tumor heterogeneity in drug-surviving cancer cells. Adv. Biosyst. 2020, 4, 1900213.
Meacham, C. E.; Morrison, S. J. Tumour heterogeneity and cancer cell plasticity. Nature 2013, 501, 328–337.
Pastushenko, I.; Brisebarre, A.; Sifrim, A.; Fioramonti, M.; Revenco, T.; Boumahdi, S.; Van Keymeulen, A.; Brown, D.; Moers, V.; Lemaire, S. et al. Identification of the tumour transition states occurring during EMT. Nature 2018, 556, 463–468.
Cheli, Y.; Guiliano, S.; Botton, T.; Rocchi, S.; Hofman, V.; Hofman, P.; Bahadoran, P.; Bertolotto, C.; Ballotti, R. Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene 2011, 30, 2307–2318.
Hölzel, M.; Bovier, A.; Tüting, T. Plasticity of tumour and immune cells: A source of heterogeneity and a cause for therapy resistance? Nat. Rev. Cancer 2013, 13, 365–376.
O’Donovan, T. R.; O’Sullivan, G. C.; McKenna, S. L. Induction of autophagy by drug-resistant esophageal cancer cells promotes their survival and recovery following treatment with chemotherapeutics. Autophagy 2011, 7, 509–524.
Huang, P.; Wang, D. L.; Su, Y.; Huang, W.; Zhou, Y. F.; Cui, D. X.; Zhu, X. Y.; Yan, D. Y. Combination of small molecule prodrug and nanodrug delivery: Amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 2014, 136, 11748–11756.
Song, H. Q.; Li, W. L.; Qi, R. G.; Yan, L. S.; Jing, X. B.; Zheng, M. H.; Xiao, H. H. Delivering a photosensitive transplatin prodrug to overcome cisplatin drug resistance. Chem. Commun. 2015, 51, 11493–11495.
Ni, X.; Jia, S. R.; Duan, X. C.; Ding, D.; Li, K. Fluorescent nanoparticles for noninvasive stem cell tracking in regenerative medicine. J. Biomed. Nanotechnol. 2018, 14, 240–256.
Carvalho, F.; George, J.; Sheikh, H. M. A.; Selvin, R. Advances in screening, detection and enumeration of Escherichia coli using nanotechnology-based methods: A review. J. Biomed. Nanotechnol. 2018, 14, 829–846.
Xue, W. T.; Di, Z. H.; Zhao, Y.; Zhang, A. P.; Li, L. L. DNAmediated coordinative assembly of upconversion hetero-nanostructures for targeted dual-modality imaging of cancer cells. Chin. Chem. Lett. 2019, 30, 899–902.
Marusyk, A.; Polyak, K. Tumor heterogeneity: Causes and consequences. Biochim. Biophys. Acta-Rev. Cancer 2010, 1805, 105–117.
Wang, H.; Dai, T. T.; Li, S. L.; Zhou, S. Y.; Yuan, X. J.; You, J. Y.; Wang, C. L.; Mukwaya, V.; Zhou, G. D.; Liu, G. J. et al. Scalable and cleavable polysaccharide nanocarriers for the delivery of chemotherapy drugs. Acta Biomater. 2018, 72, 206–216.
Wang, H.; Dai, T. T.; Zhou, S. Y.; Huang, X. X.; Li, S. Y.; Sun, K.; Zhou, G. D.; Dou, H. J. Self-assembly assisted fabrication of dextran-based nanohydrogels with reduction-cleavable junctions for applications as efficient drug delivery systems. Sci. Rep. 2017, 7, 40011.
Wang, H.; Dai, T. T.; Lu, B. L.; Li, S. L.; Lu, Q.; Mukwaya, V.; Dou, H. J. Hybrid dextran-gadolinium Nano-suitcases as high-relaxivity MRI contrast agents. Chin. J. Polym. Sci. 2018, 36, 391–398.
Dai, T. T.; Zhou, S. Y.; Yin, C. Y.; Li, S. L.; Cao, W. G.; Liu, W.; Sun, K.; Dou, H. J.; Cao, Y. L.; Zhou, G. D. Dextran-based fluorescent nanoprobes for sentinel lymph node mapping. Biomaterials 2014, 35, 8227–8235.
Zhou, S. Y.; Min, X.; Dou, H. J.; Sun, K.; Chen, C. Y.; Chen, C. T.; Zhang, Z. F.; Jin, Y. Q.; Shen, Z. L. Facile fabrication of dextran-based fluorescent nanogels as potential glucose sensors. Chem. Commun. 2013, 49, 9473–9475.
Zhou, S. Y.; Dou, H. J.; Zhang, Z. F.; Sun, K.; Jin, Y. Q.; Dai, T. T.; Zhou, G. D.; Shen, Z. L. Fluorescent dextran-based nanogels: Efficient imaging nanoprobes for adipose-derived stem cells. Polym. Chem. 2013, 4, 4103–4112.
Guo, H. Z.; Song, S.; Dai, T. T.; Li, S. L.; Dou, H. J. Trypsin-responsive near-infrared fluorescent/magnetic resonance dual-imaging composite nanospheres based on self-assembly. Acta Polym. Sin. 2018, 1127–1140. (in Chinese)
Gao, H. J.; Shi, W. D.; Freund, L. B. Mechanics of receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2005, 102, 9469–9474.
Lee, M. R.; Ju, H. J.; Kim, B. S.; Ko, Y. H.; Kim, W. S.; Kim, S. J. Isolation of side population cells in B-cell non-hodgkin’s lymphomas. Acta Haematol. 2013, 129, 10–17.
Oh, N.; Park, J. H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63.
Chakraborty, A.; Jana, N. R. Clathrin to lipid raft-endocytosis via controlled surface chemistry and efficient perinuclear targeting of nanoparticle. J. Phys. Chem. Lett. 2015, 6, 3688–3697.
Albanese, A.; Tang, P. S.; Chan, W. C. W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16.
Zeng, X. H.; Morgenstern, R.; Nyström, A. M. Nanoparticle-directed sub-cellular localization of doxorubicin and the sensitization breast cancer cells by circumventing GST-Mediated drug resistance. Biomaterials 2014, 35, 1227–1239.
Januchowski, R.; Zawierucha, P.; Andrzejewska, M.; Ruciński, M.; Zabel, M. Microarray-based detection and expression analysis of ABC and SLC transporters in drug-resistant ovarian cancer cell lines. Biomed. Pharmacother. 2013, 67, 240–245.
Fletcher, J. I.; Haber, M.; Henderson, M. J.; Norris, M. D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156.
Allikmets, R.; Schriml, L. M.; Hutchinson, A.; Romano-Spica, V.; Dean, M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998, 58, 5337–5339.
Johnatty, S. E.; Beesley, J.; Paul, J.; Fereday, S.; Spurdle, A. B.; Webb, P. M.; Byth, K.; Marsh, S.; McLeod, H.; AOCS Study Group et al. ABCB1 (MDR 1) polymorphisms and progression-free survival among women with ovarian cancer following paclitaxel/carboplatin chemotherapy. Clin. Cancer Res. 2008, 14, 5594–5601.
Kimchi-Sarfaty, C.; Oh, J. M.; Kim, I. W.; Sauna, Z. E.; Calcagno, A. M.; Ambudkar, S. V.; Gottesman, M. M. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007, 315, 525–528.
Brown, R.; Curry, E.; Magnani, L.; Wilhelm-Benartzi, C. S.; Borley, J. Poised epigenetic states and acquired drug resistance in cancer. Nat. Rev. Cancer 2014, 14, 747–753.
Beck, B.; Blanpain, C. Unravelling cancer stem cell potential. Nat. Rev. Cancer 2013, 13, 727–738.
Ottevanger, P. B. Ovarian cancer stem cells more questions than answers. Semin. Cancer Biol. 2017, 44, 67–71.
Steg, A. D.; Bevis, K. S.; Katre, A. A.; Ziebarth, A.; Dobbin, Z. C.; Alvarez, R. D.; Zhang, K.; Conner, M.; Landen, C. N. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin. Cancer Res. 2012, 18, 869–881.
Acharyya, S.; Oskarsson, T.; Vanharanta, S.; Malladi, S.; Kim, J.; Morris, P. G.; Manova-Todorova, K.; Leversha, M.; Hogg, N.; Seshan, V. E. et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 2012, 150, 165–178.
Zhang, L. Y.; Zhou, D. B.; Guan, W. C.; Ren, W. M.; Sun, W. W.; Shi, J. M.; Lin, Q. B.; Zhang, J. G.; Qiao, T. K.; Ye, Y. L. et al. Pyridoxine 5’-phosphate oxidase is a novel therapeutic target and regulated by the TGF-β signalling pathway in epithelial ovarian cancer. Cell Death Dis. 2017, 8, 3214.
Robey, R. W.; Pluchino, K. M.; Hall, M. D.; Fojo, A. T.; Bates, S. E.; Gottesman, M. M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464.
Dongre, A.; Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84.
Nieto, M. A.; Huang, R. Y. J.; Jackson, R. A.; Thiery, J. P. EMT: 2016. Cell 2016, 166, 21–45.
Sethi, G.; Sung, B.; Aggarwal, B. B. Nuclear factor-κB activation: From bench to bedside. Exp. Biol. Med. 2008, 233, 21–31.
Nakanishi, C.; Toi, M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer 2005, 5, 297–309.
Ting, A. T.; Bertrand, M. J. M. More to life than NF-κB in TNFR1 signaling. Trends Immunol. 2016, 37, 535–545.
Annibaldi, A.; Meier, P. Checkpoints in TNF-induced cell death: Implications in inflammation and cancer. Trends Mol. Med. 2018, 24, 49–65.
McIntosh, K.; Balch, C.; Tiwari, A. K. Tackling multidrug resistance mediated by efflux transporters in tumor-initiating cells. Expert Opin. Drug Metab. Toxicol. 2016, 12, 633–644.
Eum, K. H.; Lee, M. Targeting the autophagy pathway using ectopic expression of Beclin 1 in combination with rapamycin in drug-resistant v-Ha-ras-transformed NIH 3T3 cells. Mol. Cells 2011, 31, 231–238.
Shin, J. W.; Chu, K.; Shin, S. A.; Jung, K. H.; Lee, S. T.; Lee, Y. S.; Moon, J.; Lee, D. Y.; Lee, J. S.; Lee, D. S. et al. Clinical applications of simultaneous PET/MR imaging using (R)-[11C]-verapamil with cyclosporin A: Preliminary results on a surrogate marker of drug-resistant epilepsy. Am. J. Neuroradiol. 2016, 37, 600–606.
Tsouris, V.; Joo, M. K.; Kim, S. H.; Kwon, I. C.; Won, Y. Y. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol. Adv. 2014, 32, 1037–1050.
Lage, H. Therapeutic potential of RNA interference in drug-resistant cancers. Future Oncol. 2009, 5, 169–185.
Zamore, P. D.; Tuschl, T.; Sharp, P. A.; Bartel, D. P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33.
Susa, M.; Iyer, A. K.; Ryu, K.; Choy, E.; Hornicek, F. J.; Mankin, H.; Milane, L.; Amiji, M. M.; Duan, Z. F. Inhibition of ABCB1 (MDR1) expression by an siRNA nanoparticulate delivery system to overcome drug resistance in osteosarcoma. PLoS One 2010, 5, e10764.
Xia, Y. Q.; Wang, X. F.; Cheng, H.; Fang, M.; Ning, P. B.; Zhou, Y. L.; Chen, W.; Song, H. J. A polycation coated liposome as efficient siRNA carrier to overcome multidrug resistance. Colloids Surf. B Biointerfaces 2017, 159, 427–436.
Nieth, C.; Priebsch, A.; Stege, A.; Lage, H. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi). FEBS Lett. 2003, 545, 144–150.
Yadav, S.; Van Vlerken, L. E.; Little, S. R.; Amiji, M. M. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother. Pharmacol. 2009, 63, 711–722.
Zhang, X. G.; Miao, J.; Dai, Y. Q.; Du, Y Z.; Yuan, H.; Hu, F. Q. Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int. J. Pharm. 2008, 361, 239–244.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 21871180 and 81872121), the “Shuguang Program” supported by the Shanghai Education Development Foundation and the Shanghai Municipal Education Commission (No. 17SG12), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (No. SHDP201802), the Science and Technology Commission of Shanghai Municipality (No. 18520710300 and 17ZR1404100), and the Biomedical Interdisciplinary Research Foundation of SJTU (No. YG2019QNB34).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2020_2981_MOESM1_ESM.pdf
Fluorescent glycan nanoparticle-based FACS assays for the identification of genuine drug-resistant cancer cells with differentiation potential
Rights and permissions
About this article
Cite this article
Wang, C., Guan, W., Chen, R. et al. Fluorescent glycan nanoparticle-based FACS assays for the identification of genuine drug-resistant cancer cells with differentiation potential. Nano Res. 13, 3110–3122 (2020). https://doi.org/10.1007/s12274-020-2981-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2981-8