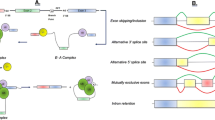
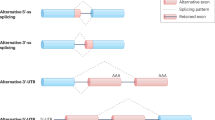
Abstract
The neuronal microtubule-associated protein tau is abnormally hyperphosphorylated and aggregated into neurofibrillary tangles in the brains of individuals with Alzheimer’s disease and related neurodegenerative disorders. The adult human brain expresses six isoforms of tau generated by alternative splicing of exons 2, 3, and 10 of its pre-mRNA. Exon 10 encodes the second microtubule-binding repeat of tau. Its alternative splicing produces tau isoforms with either three or four microtubule-binding repeats, termed 3R-tau and 4Rtau. In the normal adult human brain, the level of 3R-tau is approximately equal to that of 4R-tau. Several silent and intronic mutations of the tau gene associated with FTDP-17T (frontotemporal dementia with Parkinsonism linked to chromosome 17 and specifically characterized by tau pathology) only disrupt exon 10 splicing, but do not influence the primary sequence of the tau protein. Thus, abnormal exon 10 splicing is sufficient to cause neurodegeneration and dementia. Here, we review the regulation of tau exon 10 splicing by cis-elements and trans-factors and summarize all the mutations associated with FTDP-17T and related tauopathies. The findings suggest that correction of exon 10 splicing may be a potential target for tau exon 10 splicing-related tauopathies.
Similar content being viewed by others
References
Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of taxol. EMBO J 2003, 22: 70–77.
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 1986, 83: 4913–4917.
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 1986, 261: 6084–6089.
Alonso AD, Grundke-Iqbal I, Iqbal K. Alzheimer’s disease hyperphosphorylated tau sequesters normal tau into tangles of filaments and disassembles microtubules. Nat Med 1996, 2: 783–787.
Alonso AD, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proc Natl Acad Sci U S A 2001, 98: 6923–6928.
Alonso AD, Zaidi T, Novak M, Barra HS, Grundke-Iqbal I, Iqbal K. Interaction of tau isoforms with Alzheimer’s disease abnormally hyperphosphorylated tau and in vitro phosphorylation into the disease-like protein. J Biol Chem 2001, 276: 37967–37973.
Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci 2007, 8: 663–672.
Hernandez F, Avila J. Tauopathies. Cell Mol Life Sci 2007, 64: 2219–2233.
Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim Biophys Acta 2005, 1739: 240–250.
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989, 3: 519–526.
Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry 1992, 31: 10626–10633.
Lu M, Kosik KS. Competition for microtubule-binding with dual expression of tau missense and splice isoforms. Mol Biol Cell 2001, 12: 171–184.
Kosik KS, Orecchio LD, Bakalis S, Neve RL. Developmentally regulated expression of specific tau sequences. Neuron 1989, 2: 1389–1397.
D’Souza I, Schellenberg GD. Regulation of tau isoform expression and dementia. Biochim Biophys Acta 2005, 1739: 104–115.
Sergeant N, Delacourte A, Buee L. Tau protein as a differential biomarker of tauopathies. Biochim Biophys Acta 2005, 1739: 179–197.
Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet 2008, 40: 1413–1415.
Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, et al. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456: 470–476.
Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci 2007, 8: 819–831.
Calarco JA, Zhen M, Blencowe BJ. Networking in a global world: establishing functional connections between neural splicing regulators and their target transcripts. RNA 2011, 17: 775–791.
Kornblihtt AR, Schor IE, Allo M, Dujardin G, Petrillo E, Munoz MJ. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat Rev Mol Cell Biol 2013, 14: 153–165.
Dreyfuss G, Kim VN, Kataoka N. Messenger-RNA-binding proteins and the messages they carry. Nat Rev Mol Cell Biol 2002, 3: 195–205.
Graveley BR. Sorting out the complexity of SR protein functions. RNA 2000, 6: 1197–1211.
Caceres JF, Misteli T, Screaton GR, Spector DL, Krainer AR. Role of the modular domains of SR proteins in subnuclear localization and alternative splicing specificity. J Cell Biol 1997, 138: 225–238.
Zahler AM, Lane WS, Stolk JA, Roth MB. SR proteins: a conserved family of pre-mRNA splicing factors. Genes Dev 1992, 6: 837–847.
Manley JL, Krainer AR. A rational nomenclature for serine/arginine-rich protein splicing factors (SR proteins). Genes Dev 2010, 24: 1073–1074.
Eperon IC, Ireland DC, Smith RA, Mayeda A, Krainer AR. Pathways for selection of 5’ splice sites by U1 snRNPs and SF2/ASF. EMBO J 1993, 12: 3607–3617.
Krainer AR, Maniatis T. Multiple factors including the small nuclear ribonucleoproteins U1 and U2 are necessary for premRNA splicing in vitro. Cell 1985, 42: 725–736.
Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol 2009, 10: 741–754.
Hui J, Hung LH, Heiner M, Schreiner S, Neumuller N, Reither G, et al. Intronic CA-repeat and CA-rich elements: a new class of regulators of mammalian alternative splicing. EMBO J 2005, 24: 1988–1998.
Ngo JC, Chakrabarti S, Ding JH, Velazquez-Dones A, Nolen B, Aubol BE, et al. Interplay between SRPK and Clk/Sty kinases in phosphorylation of the splicing factor ASF/SF2 is regulated by a docking motif in ASF/SF2. Mol Cell 2005, 20: 77–89.
Colwill K, Pawson T, Andrews B, Prasad J, Manley JL, Bell JC, et al. The Clk/Sty protein kinase phosphorylates SR splicing factors and regulates their intranuclear distribution. EMBO J 1996, 15: 265–275.
Xiao SH, Manley JL. Phosphorylation-dephosphorylation differentially affects activities of splicing factor ASF/SF2. EMBO J 1998, 17: 6359–6367.
Koizumi J, Okamoto Y, Onogi H, Mayeda A, Krainer AR, Hagiwara M. The subcellular localization of SF2/ASF is regulated by direct interaction with SR protein kinases (SRPKs). J Biol Chem 1999, 274: 11125–11131.
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998, 393: 702–705.
Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A 1998, 95: 7737–7741.
D’Souza I, Schellenberg GD. Determinants of 4-repeat tau expression. Coordination between enhancing and inhibitory splicing sequences for exon 10 inclusion. J Biol Chem 2000, 275: 17700–17709.
Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta 2005, 1739: 91–103.
Qian W, Liang H, Shi J, Jin N, Grundke-Iqbal I, Iqbal K, et al. Regulation of the alternative splicing of tau exon 10 by SC35 and Dyrk1A. Nucleic Acids Res 2011, 39: 6161–6171.
D’Souza I, Poorkaj P, Hong M, Nochlin D, Lee VM, Bird TD, et al. Missense and silent tau gene mutations cause frontotemporal dementia with parkinsonism-chromosome 17 type, by affecting multiple alternative RNA splicing regulatory elements. Proc Natl Acad Sci U S A 1999, 96: 5598–5603.
Clark LN, Poorkaj P, Wszolek Z, Geschwind DH, Nasreddine ZS, Miller B, et al. Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc Natl Acad Sci U S A 1998, 95: 13103–13107.
Rizzu P, Van Swieten JC, Joosse M, Hasegawa M, Stevens M, Tibben A, et al. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet 1999, 64: 414–421.
Coulter LR, Landree MA, Cooper TA. Identification of a new class of exonic splicing enhancers by in vivo selection. Mol Cell Biol 1997, 17: 2143–2150.
D’Souza I, Schellenberg GD. Arginine/serine-rich protein interaction domain-dependent modulation of a tau exon 10 splicing enhancer: altered interactions and mechanisms for functionally antagonistic FTDP-17 mutations Delta280K AND N279K. J Biol Chem 2006, 281: 2460–2469.
Hernandez F, Perez M, Lucas JJ, Mata AM, Bhat R, Avila J. Glycogen synthase kinase-3 plays a crucial role in tau exon 10 splicing and intranuclear distribution of SC35. Implications for Alzheimer’s disease. J Biol Chem 2004, 279: 3801–3806.
Yu Q, Guo J, Zhou J. A minimal length between tau exon 10 and 11 is required for correct splicing of exon 10. J Neurochem 2004, 90: 164–172.
Kondo S, Yamamoto N, Murakami T, Okumura M, Mayeda A, Imaizumi K. Tra2 beta, SF2/ASF and SRp30c modulate the function of an exonic splicing enhancer in exon 10 of tau premRNA. Genes Cells 2004, 9: 121–130.
Wang J, Gao QS, Wang Y, Lafyatis R, Stamm S, Andreadis A. Tau exon 10, whose missplicing causes frontotemporal dementia, is regulated by an intricate interplay of cis elements and trans factors. J Neurochem 2004, 88: 1078–1090.
Wu JY, Kar A, Kuo D, Yu B, Havlioglu N. SRp54 (SFRS11), a regulator for tau exon 10 alternative splicing identified by an expression cloning strategy. Mol Cell Biol 2006, 26: 6739–6747.
Jiang Z, Tang H, Havlioglu N, Zhang X, Stamm S, Yan R, et al. Mutations in tau gene exon 10 associated with FTDP-17 alter the activity of an exonic splicing enhancer to interact with Tra2 beta. J Biol Chem 2003, 278: 18997–19007.
Gao L, Wang J, Wang Y, Andreadis A. SR protein 9G8 modulates splicing of tau exon 10 via its proximal downstream intron, a clustering region for frontotemporal dementia mutations. Mol Cell Neurosci 2007, 34: 48–58.
Shi J, Zhang T, Zhou C, Chohan MO, Gu X, Wegiel J, et al. Increased dosage of Dyrk1A alters alternative splicing factor (ASF)-regulated alternative splicing of tau in Down syndrome. J Biol Chem 2008, 283: 28660–28669.
Ding S, Shi J, Qian W, Iqbal K, Grundke-Iqbal I, Gong CX, et al. Regulation of alternative splicing of tau exon 10 by 9G8 and Dyrk1A. Neurobiol Aging 2012, 33(7): 1389–1399
Suh J, Im DS, Moon GJ, Ryu KS, de Silva R, Choi IS, et al. Hypoxic ischemia and proteasome dysfunction alter tau isoform ratio by inhibiting exon 10 splicing. J Neurochem 2010, 114: 160–170.
Broderick J, Wang J, Andreadis A. Heterogeneous nuclear ribonucleoprotein E2 binds to tau exon 10 and moderately activates its splicing. Gene 2004, 331: 107–114.
Wang Y, Gao L, Tse SW, Andreadis A. Heterogeneous nuclear ribonucleoprotein E3 modestly activates splicing of tau exon 10 via its proximal downstream intron, a hotspot for frontotemporal dementia mutations. Gene 2010, 451: 23–31.
Kar A, Havlioglu N, Tarn WY, Wu JY. RBM4 interacts with an intronic element and stimulates tau exon 10 inclusion. J Biol Chem 2006, 281: 24479–24488.
Kar A, Fushimi K, Zhou X, Ray P, Shi C, Chen X, et al. RNA helicase p68 (DDX5) regulates tau exon 10 splicing by modulating a stem-loop structure at the 5′ splice site. Mol Cell Biol 2011, 31: 1812–1821.
Patton JG, Porro EB, Galceran J, Tempst P, Nadal-Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev 1993, 7: 393–406.
Ray P, Kar A, Fushimi K, Havlioglu N, Chen X, Wu JY. PSF suppresses tau exon 10 inclusion by interacting with a stemloop structure downstream of exon 10. J Mol Neurosci 2011, 45: 453–466.
Mermoud JE, Cohen P, Lamond AI. Ser/Thr-specific protein phosphatases are required for both catalytic steps of premRNA splicing. Nucleic Acids Res 1992, 20: 5263–5269.
Kohtz JD, Jamison SF, Will CL, Zuo P, Luhrmann R, Garcia-Blanco MA, et al. Protein-protein interactions and 5’-splice-site recognition in mammalian mRNA precursors. Nature 1994, 368: 119–124.
Cao W, Jamison SF, Garcia-Blanco MA. Both phosphorylation and dephosphorylation of ASF/SF2 are required for premRNA splicing in vitro. RNA 1997, 3: 1456–1467.
Mermoud JE, Cohen PT, Lamond AI. Regulat ion of mammalian spliceosome assembly by a protein phosphorylation mechanism. EMBO J 1994, 13: 5679–5688.
Stojdl DF, Bell JC. SR protein kinases: the splice of life. Biochem Cell Biol 1999, 77: 293–298.
Lai MC, Lin RI, Tarn WY. Transportin-SR2 mediates nuclear import of phosphorylated SR proteins. Proc Natl Acad Sci U S A 2001, 98: 10154–10159.
Duncan PI, Stojdl DF, Marius RM, Scheit KH, Bell JC. The Clk2 and Clk3 dual-specificity protein kinases regulate the intranuclear distribution of SR proteins and influence pre-mRNA splicing. Exp Cell Res 1998, 241: 300–308.
Rossi F, Labourier E, Forne T, Divita G, Derancourt J, Riou JF, et al. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 1996, 381: 80–82.
Shi J, Qian W, Yin X, Iqbal K, Grundke-Iqbal I, Gu X, et al. Cyclic AMP-dependent protein kinase regulates the alternative splicing of tau exon 10: a mechanism involved in tau pathology of Alzheimer’s disease. J Biol Chem 2011, 286(16): 14639–14648
Yin X, Jin N, Gu J, Shi J, Zhou J, Gong CX, et al. Dualspecificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A) modulates serine/arginine-rich protein 55 (SRp55)-promoted Tau exon 10 inclusion. J Biol Chem 2012, 287: 30497–30506.
Kvissel AK, Orstavik S, Eikvar S, Brede G, Jahnsen T, Collas P, et al. Involvement of the catalytic subunit of protein kinase A and of HA95 in pre-mRNA splicing. Exp Cell Res 2007, 313: 2795–2809.
Patel NA, Kaneko S, Apostolatos HS, Bae SS, Watson JE, Davidowitz K, et al. Molecular and genetic studies imply Akt-mediated signaling promotes protein kinase CbetaII alternative splicing via phosphorylation of serine/arginine-rich splicing factor SRp40. J Biol Chem 2005, 280: 14302–14309.
Kentrup H, Becker W, Heukelbach J, Wilmes A, Schurmann A, Huppertz C, et al. Dyrk, a dual specificity protein kinase with unique structural features whose activity is dependent on tyrosine residues between subdomains VII and VIII. J Biol Chem 1996, 271: 3488–3495.
Gu J, Shi J, Wu S, Jin N, Qian W, Zhou J, et al. Cyclic AMPdependent protein kinase regulates 9G8-mediated alternative splicing of tau exon 10. FEBS Lett 2012, 586: 2239–2244.
Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. J Alzheimers Dis 2006, 9: 309–317.
Chen KL, Yuan RY, Hu CJ, Hsu CY. Amyloid-beta peptide alteration of tau exon-10 splicing via the GSK3beta-SC35 pathway. Neurobiol Dis 2010, 40: 378–385.
Chen C, Jin N, Qian W, Liu W, Tan X, Ding F, et al. Cyclic AMP-dependent protein kinase enhances SC35-promoted tau exon 10 inclusion. Mol Neurobiol 2014, 49(1): 615–624
Novoyatleva T, Heinrich B, Tang Y, Benderska N, Butchbach ME, Lorson CL, et al. Protein phosphatase 1 binds to the RNA recognition motif of several splicing factors and regulates alternative pre-mRNA processing. Hum Mol Genet 2008, 17: 52–70.
Ma CT, Ghosh G, Fu XD, Adams JA. Mechanism of dephosphorylation of the SR protein ASF/SF2 by protein phosphatase 1. J Mol Biol 2010, 403: 386–404.
Liu F, Gong CX. Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener 2008, 3: 8.
Rohrer JD, Paviour D, Vandrovcova J, Hodges J, de Silva R, Rossor MN. Novel L284R MAPT mutation in a family with an autosomal dominant progressive supranuclear palsy syndrome. Neurodegener Dis 2011, 8: 149–152.
Kouri N, Carlomagno Y, Baker M, Liesinger AM, Caselli RJ, Wszolek ZK, et al. Novel mutation in MAPT exon 13 (p.N410H) causes corticobasal degeneration. Acta Neuropathol 2013. Doi: 10.1007/s00401-013-1193-7.
Neumann M, Schulz-Schaeffer W, Crowther RA, Smith MJ, Spillantini MG, Goedert M, et al. Pick’s disease associated with the novel Tau gene mutation K369I. Ann Neurol 2001, 50: 503–513.
Pickering-Brown S, Baker M, Yen SH, Liu WK, Hasegawa M, Cairns N, et al. Pick’s disease is associated with mutations in the tau gene. Ann Neurol 2000, 48: 859–867.
Ros R, Thobois S, Streichenberger N, Kopp N, Sanchez MP, Perez M, et al. A new mutation of the tau gene, G303V, in early-onset familial progressive supranuclear palsy. Arch Neurol 2005, 62: 1444–1450.
Bronner IF, ter Meulen BC, Azmani A, Severijnen LA, Willemsen R, Kamphorst W, et al. Hereditary Pick’s disease with the G272V tau mutation shows predominant three-repeat tau pathology. Brain 2005, 128: 2645–2653.
de Silva R, Lashley T, Strand C, Shiarli AM, Shi J, Tian J, et al. An immunohistochemical study of cases of sporadic and inherited frontotemporal lobar degeneration using 3R- and 4R-specific tau monoclonal antibodies. Acta Neuropathol 2006, 111: 329–340.
Andreadis A. Misregulation of tau alternative splicing in neurodegeneration and dementia. Prog Mol Subcell Biol 2006, 44: 89–107.
Yoshida M. Cellular tau pathology and immunohistochemical study of tau isoforms in sporadic tauopathies. Neuropathology 2006, 26: 457–470.
Hogg M, Grujic ZM, Baker M, Demirci S, Guillozet AL, Sweet AP, et al. The L266V tau mutation is associated with frontotemporal dementia and Pick-like 3R and 4R tauopathy. Acta Neuropathol 2003, 106: 323–336.
Glatz DC, Rujescu D, Tang Y, Berendt FJ, Hartmann AM, Faltraco F, et al. The alternative splicing of tau exon 10 and its regulatory proteins CLK2 and TRA2-BETA1 changes in sporadic Alzheimer’s disease. J Neurochem 2006, 96: 635–644.
Yasojima K, McGeer EG, McGeer PL. Tangled areas of Alzheimer brain have upregulated levels of exon 10 containing tau mRNA. Brain Res 1999, 831: 301–305.
Chambers CB, Lee JM, Troncoso JC, Reich S, Muma NA. Overexpression of four-repeat tau mRNA isoforms in progressive supranuclear palsy but not in Alzheimer’s disease. Ann Neurol 1999, 46: 325–332.
Boutajangout A, Boom A, Leroy K, Brion JP. Expression of tau mRNA and soluble tau isoforms in affected and nonaffected brain areas in Alzheimer’s disease. FEBS Lett 2004, 576: 183–189.
Espinoza M, de Silva R, Dickson DW, Davies P. Differential incorporation of tau isoforms in Alzheimer’s disease. J Alzheimers Dis 2008, 14: 1–16.
Sengupta A, Novak M, Grundke-Iqbal I, Iqbal K. Regulation of phosphorylation of tau by cyclin-dependent kinase 5 and glycogen synthase kinase-3 at substrate level. FEBS Lett 2006, 580: 5925–5933.
Alonso AD, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem 2004, 279: 34873–34881.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qian, W., Liu, F. Regulation of alternative splicing of tau exon 10. Neurosci. Bull. 30, 367–377 (2014). https://doi.org/10.1007/s12264-013-1411-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-013-1411-2