Abstract
Association between cancer and myositis has been extensively reported and malignancy is a potentially life-threating complication in myositis. In this retrospective study authors give an overview of Hungarian cancer-associated myositis (CAM) patients treated at a single centre managing 450 myositis patients. All patients were diagnosed according to Bohan and Peter. Statistical analysis of disease onset, age, sex, muscle, skin and extramuscular symptoms, muscle enzymes, presence of antibodies, treatment and prognosis was performed. 43 patients could be considered as having CAM. 83.72% had cancer within one year of diagnosis of myositis. Most common localizations were ductal carcinoma of breast and adenocarcinoma of lung. Significant differences were observed between CAM and the non-CAM control patients: DM:PM ratio was 2.31:1 vs. 0.87:1, respectively (p = 0.029), age at diagnosis was 56.60 ± 12.79 vs. 38.88 ± 10.88 years, respectively (p < 0.001). Tumour-treatment was the following: surgical removal in 55.81%, chemotherapy in 51.1%, radiotherapy in 39.53%, hormone treatment in 18.6%, combination therapy in 51.16% of patients. Muscle enzyme levels of patients undergoing surgery were significantly reduced after intervention. 36 patients died (83.72%); 25 DM (83.33%) and 11 PM patients (84.62%); 5 years survival was 15.4% for PM and 27.5% for DM. This study demonstrates that DM, distal muscle weakness, asymmetric Raynaud’s phenomenon, older age, ANA-negativity are risk factors for developing malignancy and polymyositis patients have less chance of long-lasting survival. It is very important to think about cancer and follow every single myositis patient in the clinical routine because survival rate of CAM is very poor.
Similar content being viewed by others
Introduction
Idiopathic inflammatory myopathies (IIMs) are systemic, chronic, autoimmune diseases, characterised by symmetrical, proximal muscle weakness. The IIMs fall into six clinico-pathological categories: (1) dermatomyositis (DM; juvenile, adult), (2) polymyositis (PM), (3) overlap syndromes (OM), (4) cancer-associated myositis (CAM), (5) inclusion body myositis (IBM), and (6) focal and diffuse myositis, including necrotizing autoimmune myopathy (NAM) [1,2,3,4,5,6,7,8,9,10,11]. The link between myositis and cancer was originally noticed in the early 1900’s [12], also by Bohan and Peter in their classification in 1975 [13], and has been than proved by large population-based studies [14]. Modern epidemiologic works have provided strong support for this association and evidence that it may also be true for PM, but the risk is lower than that for DM patients [15]. This well-recognised association between IIM and malignancy is a remarkable complication contributing to increased mortality in myositis. While the exact pathogenesis of CAM remains unclear, a paraneoplastic nature was assumed in the majority of these patients because cancer diagnosis and myositis onset seemed to temporally coincide [16]. The discovery of myositis-associated antibodies (MAAs) and myositis-specific antibodies (MSAs) led to the development of the clinico-serological classification and the recently discovered anti-TIF1gamma antibodies seem to be strong related to malignancy [17, 18]. The recent publication of the European League Against Rheumatism and American College of Rheumatology (EULAR/ACR) classification criteria for IIM [19, 20] was a big step forward because it makes the everyday work of the physician easier. Despite its many advantages this classification criteria does not contain any specific factor that can help in the recognition of CAM patients. There is limited evidence for specific treatment strategies in IIM and this also applies for CAM [21]. It is still of primary importance to diagnose these complicated cases as early as possible because the possibility of survival increases with early identification and therapy.
Aims
In this retrospective study authors aimed to give a retrospective overview of Hungarian myositis patients with malignancy treated at a single centre. At the Division of Clinical Immunology, Department of Medicine, University of Debrecen, Hungary, 450 myositis patients have been treated since the end of the 1980’s, 304 patients with PM, OM, IBM or NAM and 146 patients with DM. Our aim was to study the clinical, immunological and therapeutic characteristics of myositis cases associated with cancer from the last 3 decades.
Methods
Data Collection
We retrospectively collected data from the University’s computerized patient record system, called eMedSolution. All selected myositis patients had a definitive or probable diagnosis for myositis (muscle weakness, high muscle enzyme levels, plus positive EMG and / or positive muscle biopsy in polymyositis or skin symptoms in dermatomyositis, according to Bohan and Peter). No juvenile DM/PM cases were selected. All myositis patients that had cancer during their life were primarily enrolled to the study. Based on these, 60 cases could be identified. Analysis of following epidemiologic, clinical, laboratory and therapeutic data were performed: age, sex, muscle symptoms, skin symptoms, extramuscular symptoms, serum level of creatine kinase, lactate dehydrogenase, presence or absence of MSAs or MAAs, drugs used for the treatment of cancer and myositis, and prognosis of patients.
Detection of Antibodies
Immunoserological analyses, which were performed at the Department of Laboratory Medicine, University of Debrecen, included tests for the following autoantibodies. Antinuclear antibodies (ANA) were determined by indirect immunofluorescence on HEp-2 cells (Viro-Immun Labordiagnostika GmbH, Oberursel, Deutschland); ANA positivity was assessed at 1:40 dilution. Anti-Scl70, anti-Sm/RNP were determined in all patients by ELISA (Hycor Biomedical Inc., Garden Grove, CA, USA). Anti-Jo-1, anti-Mi-2, anti-Pm-Scl, anti-Ku antibodies were detected by membrane-fixed immuno-blot (Orgentec Diagnostika GmbH, Mainz, Deutschland). Anti-SSA and anti-SSB were determined by ELISA (Hycor Biomedical Inc., Garden Grove, CA, USA), as well as anti-U1RNP (Orgentec Diagnostica GmbH, Mainz, Deutschland). These commercially available methods were used following the manufacturer’s protocol.
Diagnosis of Clinical Parameters, Muscular and Internal Organ Involvement
EMG was performed using the Buchtal-method at Department of Neurology, University of Debrecen. Muscle biopsies were performed by surgeons and analysed by a neuropathologist. Pulmonary fibrosis was defined as present by radiographic findings and pulmonary function tests (spirometry, DLCO). Dysphagia was diagnosed by barium radiography of the oesophagus. Cardiac involvement was encoded in case of pericarditis, myocarditis, conduction disturbances, myocardial ischemia and recurrent arrhythmia; it was assessed by ECG, two-dimensional and Doppler echocardiography. Imaging studies (US, X-ray, CT, HRCT, MRI, PET-scan) were done, if needed, at the Department of Radiology, University of Debrecen.
Statistics
To compare groups with categorical data Pearson Chi-square (χ2) test was used. To compare groups with small number of cases Fisher’s exact test was used. Statistical analysis was made using SPSS 20.0 statistical software. When creating a small group of patients as control group random number generator had been used. During statistical analysis, the P value less than 0.05 was regarded as statistically significant.
Results
There are no clear literature data on when to consider a case as a CAM patient because the exact characteristics of the timely association of malignant disease and myositis are not known. Based on the previous work of our group [22] plus relying on data from other population studies [23], we evaluated the myositis as „tumour associated” in the following cases: 1. if the tumour was diagnosed within two years before muscle symptoms; 2. if the cancer process has been diagnosed within three years after the onset of myositis symptoms. Accordingly, out of a total of 60 patients who have ever had cancer, a total of 43 patients could be considered as having CAM (Fig. 1.). Hereinafter we deal with these 43 patients. In seven patients symptoms of myositis and cancer appeared simultaneously (±3 months); in another 29 patients cancer was diagnosed ±1 year of myositis onset. This means that 83.72% of all patients had cancer within one year of the diagnosis of myositis. DM:PM ratio was 2.31:1, with 30 DM and 13 PM patients. Age at the diagnosis of CAM was 56.60 ± 12.79 (55.9 ± 13.71 for DM and 58.23 ± 10.71 for PM patients). Female:male ratio was 2.07:1, with 29 women and 14 men, for DM patients 2.33:1 and for PM patients 1.6:1.
In our opinion it is important to know when these cases were diagnosed. If we divided the 29 years between 1990 and 2018 into 5-year periods, the number of patients in each time interval was as follows: 1990–1994: one patient, 1995–1999: six, 2000–2004: twelve, 2005–2009: thirteen, 2010–2014: nine, 2015-nowadays: two patients.
One of the most important questions is the type of cancers that were associated to the myositis. These data can be followed on Table 1. The two most common anatomical localizations were breast and lung while the most common histological types were ductal carcinoma of the breast and adenocarcinoma in different localizations.
To compare some of the most important characteristics of CAM patients and “simple” myositis patients we generated a control group. Between 1990 and 2018, we also created six “5-year intervals”. Out of the 390 non-CAM patients, 43 patients were randomly selected using a computer (random number generator); there were as many patients in each interval as it was by the CAM group, representing the whole non-cancer associated myositis population. In this group, no patient had ever malignancy. The DM:PM ratio was 0.87:1. Age at diagnosis of myositis was 38.88 ± 10.88, female:male ratio was 2.31:1 with 30 women and 13 men. These data are summarized in Table 2. We can see that there was a significant difference between the two groups in the case of the DM:PM ratio and the disease onset. Most important clinical, serological and therapeutic data of these two groups are summarized and compared in Table 3.
Some notable results of symptoms in the CAM group were for example the relative high number of distal muscle weakness. We underline the clinical importance of skin symptoms in the patients associated with cancer. Most important extramuscular manifestations in the CAM group were joint involvement and oesophagus involvement. Only 18.6% and 23.26% of patients with CAM had any MSAs or MAAs. All patients received steroids but other immunosuppressant drugs were not widely used in this group of patients.
Another interesting question is to explain what levels of muscle enzymes have occurred in our patients. In CAM patients, at onset of muscle symptoms, the mean value of creatine kinase levels was 3269.34 ± 4306.35 IU/L (2715.95 ± 3564.52 IU/L for DM and 4078.15 ± 5238 IU/L for PM patients), and the mean value of lactate dehydrogenase was 1019 ± 760.69 IU/L (1096.67 ± 752.07 IU/L for DM and 894.31 ± 788.16 IU/L for PM patients). In the control group, at onset of muscle symptoms, these mean values were 4543.16 ± 4542.09 IU/L for CK and 1248.58 ± 3222.55 IU/L for LDH (statistically significant higher than those in CAM patients: p values 0.048 and 0.021, respectively). CK and LDH of patients undergoing surgery were significantly reduced after intervention. CK just before surgery was 4297.20 ± 5584.24 IU/L and after surgery was 1144.43 ± 887.82 IU/L (p = 0.006); LDH right before surgery was 1126.38 ± 828.61 IU/L and after surgery was 653.19 ± 620.82 (p = 0.027).
We should also mention the oncological treatment of these 43 patients. In 24 cases (55.81%) surgical removal of the tumour occurred, 22 patients (51.1%) received chemotherapy, 17 patients (39.53%) received radiotherapy, 8 patients (18.6%) got hormone treatment, 5 extremely serious cases (11.62%) did not receive any anti-tumour treatment. A total of 22 patients (51.16%) received combination therapy (two, three or four types of treatment).
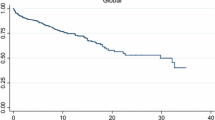
This question leads us to another important issue: survival of CAM patients. All together 36 of the 43 patients died (83.72%); 25 DM (83.33%) and 11 PM patients (84.62%). According to the statistical analysis the mean survival for the 43 CAM patients were 54.85 (29.44–80.26; 95% CI) months, the survival rates at one and at five years from diagnosis were 55.5% and 23.4%, respectively. These data for the 30 DM patients out of the 43 CAM patients are: mean survival 57.55 (25.06–89.04; 95% CI) months, the survival rates at one and at five years from diagnosis were 49.4% and 27.5%, respectively. The appropriate data for the 13 PM patients out of the 43 CAM patients are: mean survival 37.85 (18.83–56.86; 95% CI) months, the survival rates at one and at five years from diagnosis were 69.2% and only 15.4%, respectively. Survival rates can be followed on Fig. 2. Median survival of the 43 CAM patients were 16.00 (4.54–24.46; 95% CI) months. As mentioned earlier, the disease was extremely fulminant in five patients. In four cases 2–5 months after severe myositis symptoms, lung cancer was confirmed. Their survival was only 1–2 months after the diagnosis of the tumour; in their case, the cause of death was the following: respiratory failure due to respiratory muscle involvement; severe bronchitis; pneumonia; progression of the tumour process due to brain metastases. In another case, severe DM symptoms were associated with metastatic pancreatic cancer, and the patient died of liver failure. Cause of death of other CAM patients, apart from the above mentioned, were among others ileus, renal failure, tumour progression, metastases or sepsis.
Discussion
Our department has decades of experience in the field of healing of patients with myositis. After 29 years of follow-up, we believe that it can be stated that cancer cases occurring two years before or three years after the diagnosis of myositis can be considered as CAM. In this study, more than 80% of the cancers were diagnosed within one year of onset of muscle symptoms which means that it is very important to think about the possibility of cancer at every single myositis patient. Out of the 450 myositis patients there were 43 CAM patients (9.56%); this means that one in every ten myositis patient is a CAM patient. In an Italian cohort it was 17% [23]. In our whole myositis population there were 30 DM-CAM (out of 146 patients; 20.55%) and 13 PM-CAM (out of 304 patients; 4.28%) patients. This reflects that: both DM and PM are associated with increased risk of malignancy but dermatomyositis is more associated with any type of cancer than polymyositis. This is similar to the results of other workgroups [24]. In a study from northern China, the frequency of malignancy in DM patients was 17.99%, similar as our result [25]. Some other interesting data of this Chinese cohort also should be discussed here. 69.77% of malignancy was diagnosed within the first year before or after the onset of myositis; this is a little bit lower than our result. The most frequent histology was adenocarcinoma but they found that lung cancer was the most frequent localisation. According to the most recent demographic data of the cancer registries (2006–2015) in Hungary the lung (males) and breast (females) cancers are at first place in cancer-morbidity hierarchy [26]. This is in harmony with our results. Just like us, other workgroups also detected other atypical types of tumour-localisation in CAM patients: ovarian cancer [27], colorectal cancer [28], haematological malignancy [29], cervical cancer [30], pancreatic tumour [31], carcinoma of the penis [32] or prostate cancer [33].
Comparing the CAM patients to the control group, as seen in Table 2., older age can be a risk factor for malignancy in myositis patients. 56.6 years at disease onset is the same as found by Sellami et al. in 2018 [34]. In our studied population there was no significant difference in female:male ratio. In contrast, a meta-analysis from China found that male sex increases the risk of malignancy [35]. As seen in Table 3., the frequency of ANF positivity in CAM patients was statistically significant lower than in patients without malignancy. The same result was concluded by Hoesly et al. in their study in 2018 [36]. Skin symptoms were more frequent in the CAM group this is because DM:PM ratio was significantly higher in these patients. There is data in the international literature that distal muscle weakness is more frequent in malignancy-associated cases; 16.28% of this group had this kind of muscle symptom and this is a significant difference between the two groups. Perhaps it also can be a warning sign for cancer. The frequency of Raynaud’s phenomenon was significantly lower in the malignancy-related group. In a high percentage of cases it was asymmetric and that can be the first sign of malignancy [37]. There was no significant difference between the two populations in the frequency of extramuscular manifestations (arthralgia, dysphagia, heart involvement and lung fibrosis). A meta-analysis found that malignancy was associated with a reduced risk of developing ILD [38]. Another systemic review said that several factors were associated with lower risk of malignancy, including the presence of ILD, arthritis/arthralgia or anti-Jo-1 antibody [39]. As seen in Table 3., we cannot strengthen these data. But Lu et al., as well as our workgroup, found that the Raynaud’s phenomenon is associated with lower-than-average risk for malignancy [39].
Despite anti-tumour therapy, survival rate of our CAM patients was very poor. The survival rate of primary PM and DM group at 5 years was significantly higher (100% and 100%), compared with the CAM-patient group, where survival was 15.4% for PM and 27.5% for DM. Although the risk in PM patients is lower to have cancer, there is less chance of long-lasting survival. Neri et al. found that the chance of long-lasting survival is higher in PM patients [23]. Regarding anti-myositis therapy immunosuppressant drugs were less frequently used in CAM patients than in simple myositis patients. Despite the widely studied association between myositis and cancer the best strategy for diagnosing and treat cancer in IIM patients is lacking [40].
Conclusion
Authors underline the clinical importance of the fast diagnosis and the best possible therapy in patients with cancer-associated myositis. According to the results of this retrospective review the risk of malignancy is present in both genders and higher age groups and is highest in the first year before or after onset of myositis. Patients with DM have a higher incidence of malignancy than patients with PM and both groups have a poor prognosis. Adults should be evaluated for malignancy at diagnosis, followed by long-term surveillance.
References
Mastaglia FL, Phillips BA (2002) Idiopathic inflammatory myopathies: epidemiology, classification, and diagnostic criteria. Rheum Dis Clin N Am 28:723–741
Sun C, Lee JH, Yang YH, Yu HH, Wang LC, Lin YT et al (2014) Juvenile Dermatomyositis: a 20-year retrospective analysis of treatment and clinical outcomes. Pediatr Neonatol S1875-9572:00093
Luo YB, Mastaglia FL (2014) Dermatomyositis, polymyositis and immune-mediated necrotising myopathies. Biochim Biophys Acta S0925-4439:00162–00168
Catalán M, Selva-O’Callaghan A, Grau JM (2014) Diagnosis and classification of sporadic inclusion body myositis (sIBM). Autoimmun Rev 13:363–366
Neri R, Barsotti S, Iacopetti V, Tripoli A, d’Ascanio A, Tavoni AG et al (2014) Clinically amyopathic dermatomyositis: analysis of a monocentric cohort. J Clin Neuromuscul Dis 15:157–160
Dankó K, Ponyi A, Molnar AP, András C (2009) Constantin T. Paraneoplastic myopathy Curr Opin Rheumatol 21:594–598
Prieto-González S, Grau JM (2014) Diagnosis and classification of granulomatous myositis. Autoimmun Rev 13:372–374
Selva-O'Callaghan A, Trallero-Araguás E, Grau JM (2014) Eosinophilic myositis: an updated review. Autoimmun Rev 13:375–378
Yan J, Wu P (2014) Idiopathic orbital myositis. J Craniofac Surg 25:884–887
Michaelides SA, Passalidou E, Bablekos GD, Aza E, Goulas G, Chorti M et al (2014) Cavitating lung lesion as a manifestation of inflammatory tumor (pseudotumor) of the lung: a case report and literature review. Am J Case Rep 15:258–265
Vajsar J, Jay V (1996) Babyn P. Infantile myositis presenting in the neonatal period 18:415–419
Stertz G (1916) Polymyositis. Berl Klin Wochenschr 53:489
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292:344–347
Chow WH, Gridley G, Mellemkjaer L et al (1995) Cancer risk following polymyositis and dermatomyositis: a nationwide cohort study in Denmark. Cancer Causes Control 6:9–13
Zahr ZA, Baer AN (2011) Malignancy in myositis. Curr Rheumatol Rep 13:208–215
Yang H, Peng Q, Yin L, Li S, Shi J, Zhang Y et al (2017) Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: a large longitudinal cohort study. Arthritis Res Ther 19:259
Hida A, Yamashita T, Hosono Y, Inoue M, Kaida K, Kadoya M et al (2016) Anti-TIF1-γ antibody and cancer-associated myositis: a clinicohistopathologic study. Neurology 87:299–308
Trallero-Araguás E, Labrador-Horrillo M, Selva-O'Callaghan A, Martínez MA, Martínez-Gómez X, Palou E et al (2010) Cancer-associated myositis and anti-p155 autoantibody in a series of 85 patients with idiopathic inflammatory myopathy. Medicine (Baltimore) 89:47–52
Lundberg IE, Tjarnlund A, Bottai M, Werth VP, Pilkington C, Visser M et al (2017) European league against rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 76:1955–1964
Leclair V, Lundberg IE (2018) New myositis classification criteria-what we have learned since Bohan and Peter. Curr Rheumatol Rep 20:18
Tieu J, Lundberg IE, Limaye V (2016) Idiopathic inflammatory myositis. Best Pract Res Clin Rheumatol 30:149–168
András C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P et al (2008) Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol 35:438–444
Neri R, Barsotti S, Iacopetti V, Iacopetti G, Pepe P, d'Ascanio A et al (2014) Cancer-associated myositis: a 35-year retrospective study of a monocentric cohort. Rheumatol Int 34:565–569
Qiang JK, Kim WB, Baibergenova A, Alhusayen R (2017) Risk of malignancy in Dermatomyositis and Polymyositis. J Cutan Med Surg 21:131–136
Liu Y, Xu L, Wu H, Zhao N, Tang Y, Li X et al (2018) Characteristics and predictors of malignancy in dermatomyositis: analysis of 239 patients from northern China. Oncol Lett 16:5960–5968
Menyhárt O, Fekete JT, Győrffy B (2018) Demographic shift disproportionately increases cancer burden in an aging nation: current and expected incidence and mortality in Hungary up to 2030. Clin Epidemiol 10:1093–1108
Flynn M, Ottaway Z, Kaur J, Waters J (2018) Three differently timed presentations of dermatomyositis associated with advanced ovarian cancer. BMJ Case Rep pii: bcr-2017-222627
Gkegkes ID, Minis EE, Iavazzo C (2018) Dermatomyositis and colorectal cancer: a systematic review. Ir J Med Sci 187:615–620
Lim CH, Tseng CW, Lin CT, Huang WN, Chen YH, Chen YM et al (2018) The clinical application of tumor markers in the screening of malignancies and interstitial lung disease of dermatomyositis/polymyositis patients: a retrospective study. SAGE Open Med 6:2050312118781895
Kumar S, Mahajan BB, Kaur S, Singh A (2014) Paraneoplastic dermatomyositis with carcinoma cervix: a rare clinical association. Case Rep Dermatol Med 2014:836246
Piovesan DM, da Silva VD, Reichel CL, Baú P, Hoefel Filho JR, Staub HL (2010) Neuroendocrine pancreatic tumor and dermatomyositis. Pancreas 39:684
Lalla SC, Aldridge RD, Tidman MJ (2001) Carcinoma of the penis presenting with dermatomyositis. Clin Exp Dermatol 26:558
Mooney CJ, Dunphy EJ, Stone B, McNeel DG (2006) Identification of autoantibodies elicited in a patient with prostate cancer presenting as dermatomyositis. Int J Urol 13:211–217
Sellami K, Mseddi M, Snoussi M, Gharbi H, Frikha F, Salah RB et al (2018) Malignancy in a retrospective cohort of 17 patients with Dermatomyositis or Polymyositis in southern Tunisia. Rom J Intern Med 56:243–249
Wang J, Guo G, Chen G, Wu B, Lu L, Bao L (2013) Meta-analysis of the association of dermatomyositis and polymyositis with cancer. Br J Dermatol 169:838–847
Hoesly PM, Sluzevich JC, Jambusaria-Pahlajani A, Lesser ER, Heckman MG, Abril A (2018) Association of antinuclear antibody status with clinical features and malignancy risk in adult-onset dermatomyositis. J Am Acad Dermatol
Sutić A, Gračanin G, Morović-Vergles J (2014) Raynaud's phenomenon - first sign of malignancy: case report. Acta Med Croatica 68:295–298
Zhang L, Wu G, Gao D, Liu G, Pan L, Ni L et al (2016) Factors associated with interstitial lung disease in patients with Polymyositis and Dermatomyositis: a systematic review and meta-analysis. PLoS One 11:e0155381
Lu X, Yang H, Shu X, Chen F, Zhang Y, Zhang S et al (2014) Factors predicting malignancy in patients with polymyositis and dermatomyostis: a systematic review and meta-analysis. PLoS One 9:e94128. https://doi.org/10.1371/journal.pone.0094128
Selva-O'Callaghan A, Martinez-Gómez X, Trallero-Araguás E, Pinal-Fernández I (2018) The diagnostic work-up of cancer-associated myositis. Curr Opin Rheumatol 30:630–636
Funding
Open access funding provided by University of Debrecen (DE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
András, C., Bodoki, L., Nagy-Vincze, M. et al. Retrospective Analysis of Cancer-Associated Myositis Patients over the Past 3 Decades in a Hungarian Myositis Cohort. Pathol. Oncol. Res. 26, 1749–1755 (2020). https://doi.org/10.1007/s12253-019-00756-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-019-00756-4