Abstract
In New Zealand, potato crop yields of 90 t ha−1 are achievable but at 55 t ha−1, the average is becoming economically unsustainable. In 2012/13, a grower-initiated survey found that Rhizoctonia solani and Spongospora subterranea (soil-borne pathogens) and soil compaction were widespread in 11 Canterbury potato crops. Targeted areas in these crops had measured yield losses of 0 (healthy plants) – 42 (diseased, resource constrained plants) t ha−1, limiting the farmer-measured field yield to a 56 t ha−1 average. In 2014/15, growth, water use and health of three contrasting crops were measured. Near-potential yield was achieved in one crop when disease incidence and severity were low and resources adequate. The other two crops yielded less than potential; one had an inadequate water supply and the other widespread soil-borne disease. Any suspected links between soil-borne disease, soil compaction and yield were further investigated by using them as factors in replicated experiments reported elsewhere.
Resumen
En Nueva Zelanda, son alcanzables los rendimientos de los cultivos de papa de 90 t ha -1, pero a 55 t ha -1, el promedio se está volviendo económicamente insostenible. En 2012/13, en una encuesta iniciada por los productores se encontró que Rhizoctonia solani y Spongospora subterranea (patógenos del suelo) y la compactación del suelo estaban muy extendidas en 11 siembras de papa de Canterbury. Las áreas objetivo en estos cultivos habían medido pérdidas de rendimiento de 0 (plantas sanas) – 42 (plantas enfermas, con recursos limitados) t ha -1, limitando el rendimiento del campo medido por el agricultor a un promedio de 56 t ha -1. En 2014/15, se midió el crecimiento, el uso del agua y la sanidad de tres cultivos contrastantes. Se logró un rendimiento casi su potencial en un cultivo cuando la incidencia y la gravedad de la enfermedad eran bajas y los recursos adecuados. Los otros dos cultivos rindieron menos de lo potencial; uno tenía un suministro de agua inadecuado y el otro tuvo enfermedades del suelo generalizadas. Cualquier sospecha de vínculos entre las enfermedades del suelo, la compactación del suelo y el rendimiento se investigó posteriormente utilizándolos como factores en experimentos repetidos reportados en otras partes.
Similar content being viewed by others
Introduction
Potatoes are the third most widely grown food crop in the world, after rice and wheat, and are very important for human nutrition. Compared with cereals, potatoes have the potential to produce more food per hectare while using less water (CIP 2018). Potato yields in developing countries (many farmers with small land areas) are well below potential (FAO 2008; Roberto et al. 2014; Tolno et al. 2016; Gebru et al. 2017; Harahagazwe et al. 2018). In many developed countries, large-scale commercial yields (particularly of processing potatoes) are also often well short of potential (Haverkort and Struik 2015), reducing land-use efficiency and the ability of farmers to maintain profitable businesses. New Zealand is no exception.
Potato crops in carefully managed experiments and in some farmers’ fields in Canterbury, New Zealand, have regularly achieved yields of approximately 90 t ha−1 (Jamieson et al. 2003). However, at the time of the study, potato farmers in this region reported that their yields had remained at 50 to 60 t ha−1 for 10 years, in spite of costly investments in the latest irrigation, cultivation and pest control technologies.
This paper is a prequel to an already published paper (Sinton et al. 2020) about the causes of yield decline in New Zealand potato crops. In the southern winter of 2012, growers and potato industry representatives called a meeting with potato agronomists and pathologists to initiate a project to seek the causes of the recognized yield gap and suggest solutions that would lead to its reduction. Out of the discussion came a plan for an intensive survey of multiple crops in the coming season to investigate management, soil and pathology issues, and to generate hypotheses as to probable causes of the yield gap. Two years later three growers, coordinating with a processor representative, were testing new soil mapping and aerial crop-monitoring technologies as management aids. They invited us to monitor the growth, health, and water use of the crops at intervals throughout the growing season. The time-course and magnitude of potato biomass accumulation, where not constrained by drought, nutrient shortage, or weeds, pest and diseases, is highly predictable. A particularly effective way of making the predictions is through the use of well-verified computer simulation model that captures the main determinants of growth and development. The Potato Calculator (Jamieson et al. 2003) is a nitrogen and irrigation management guide that has at its heart a simulation model that predicts biomass accumulation as a function of the amount of solar radiation intercepted by the crop canopy (Monteith 1977; Jamieson et al. 2003). The model calculates daily increase of green area index (GAI, area of green tissue per unit ground area), and the life duration of the crop as a time-integral of temperature. For instance, the lifetime of a Russet Burbank crop is close to 2000 °C days (base 0 °C; Jamieson et al. 2003); for the first half of the lifetime GAI increase linearly in thermal time, and for the second half decreases linearly to zero. At maturity, the harvest index of potatoes approaches unity, so most of the remaining biomass is made up of tubers. It follows that anything that reduces either size or duration of the canopy will result in reduced total biomass, and hence total tuber yields. In a healthy, well-managed potato crop the GAI will be super-optimal for light interception; a GAI of 4 intercepts 91% of the incident light, whereas increasing the GAI to 6 increases the proportion of radiation intercepted by only an additional 6%, whereas the N-content of the canopy is increased by 50%. This is because another main function of the crop canopy is to store enough N to keep feeding the growing tubers, which have a necessary but variable requirement for N, (Jamieson et al. 2008). A high proportion of the N is in leaf tissues. Increasing GAI from 4 to 6.5 increases N stored in the leaves by 50 kg ha−1. When labile sources of N in stems and soil are exhausted, the green area is sacrificed to supply the tubers, or in the canopy expansion phase, canopy expansion will cease if there is insufficient N. Daily evapotranspiration and water stress effects are calculated. A detailed description and report of experimental tests is given in Jamieson et al. (2003). The model was tested in a range of experimental conditions and was shown to be an accurate predictor in those situations where either growth is unconstrained, or limitations are associated with shortages of water or N. In the context of this study, Potato Calculator calculations give an upper bound for production. The study is designed to identify causes where production is at less than the potential rate.
Yield reductions, or the yield gap between achieved production and the potential (unconstrained) yield calculated from the Potato Calculator might be associated with either fertility issues, foliar diseases, insect damage, root diseases or soil physical problems. Some of these are less likely than others. In separate experiments in Canterbury potato crops, Michel et al. (2013) and Reid et al. (2016) showed that there was little effect on production of increasing the applied nutrients by a factor of two. Reid et al. (2011) earlier estimated yield losses in Canterbury of less than 8%, due to nutrient deficiency, were mainly caused by insufficient nitrogen nutrition. These losses are substantially less than the observed yield gap of 35-45%. These studies ruled out inadequate supply of major nutrients as a major cause of yield losses - at least at current levels of production. Hence nutrient shortage was unlikely to be a major contributing cause of the yield gap.
Potato yield reductions may be associated with many conditions and potato diseases, some are more likely than others to be the cause major and widespread yield reductions. Canterbury potato farmers practice effective foliar disease management by routinely spraying their crops to control insects (aphids and the tomato potato psyllid) and the foliar diseases early blight, (caused by Alternaria solani), and late blight (Phytophthora infestans). Verticillium wilt (Verticillium dahliae or Verticillium albo-atrum), black dot (Colletotrichum coccodes), white mold (Sclerotinia sclerotiorum), black leg (Pectobacterium atrosepticum), and diseases caused by potato viruses and nematodes (Pratylenchus and Meloidogyne spp.) occur sporadically; these are unlikely to be the causes of persistent and widespread yield reductions. Although growers apply registered pesticides in-furrow at planting, attempting to control soil-borne diseases, the seed tuber- and soil-borne diseases Rhizoctonia stem canker and Spongospora root galling and tuber powdery scab are widespread and not well controlled (McCulloch et al. 2018). Root diseases caused by Spongospora subterranea have been associated with reduced water and nutrient uptake by potato plants, and reduced productivity (Falloon et al. 2016). It is therefore likely that the major causes of poor potato yields lie below the soil surface.
Research in Canterbury between 2002 and 2005 (Jamieson et al. 2006) showed that potato yields were seldom limited by nitrogen supply, but poorly scheduled irrigation could cause yield reductions of 5 to 15 t ha−1. A similar project in the North Island of New Zealand from 2005 to 2008 (Sinton et al. 2009) estimated that yield losses from 5 to 48 t ha−1 were associated with inadequate water supply and subsoil compaction. Carter and Sanderson (2001) found that length of rotation had substantial effects on Rhizoctonia disease pressure, and also on degradation of soil physical structure. Soil compaction slows root penetration and hence restricts access to soil water (Stalham et al. 2007). However, few of these studies addressed the potential contribution of soil-borne diseases or soil compaction to yield losses. Any factor that interferes sufficiently with the function of root systems effectively reduces access to water and N, and will mimic the effects of nutrient shortage and drought. The clearest symptom of damaged root function is canopy loss and cessation of growth, and thus reduced yield (Jamieson 1985; Martin et al. 1991; Jamieson et al. 2003).
The objective of this study was firstly to identify disease and soil physical factors associated with yield losses over a large number of potato crops (11) in Canterbury, New Zealand. Then, based on the findings from the first year, case studies of three contrasting crops were made in a following year. These involved more intensive monitoring. The objective of these was to determine associations between major yield-limiting factors and the growth and yield of the crops. The knowledge would be used to generate hypotheses about the probable cause of those losses. Later experimental tests of those hypotheses were reported by Sinton et al. (2020). From this, crop management plans were devised to limit or eliminate yield-limiting factors and maximize yields from intensively managed potato crops.
A further objective of this paper was to show that a community-led investigation that involved researcher input could lead to more tightly specified hypotheses for experimental testing, and ultimately to behavior change amongst growers.
Materials and Methods
Potato Crop Survey
Eleven processing potato crops (areas from 7 to 80 ha) were selected in the spring of 2012. All sites were on farms on the coastal plain of Canterbury in the South Island of New Zealand, between latitudes 43.8° and 44.4° S. These were planted (and managed) by the farmers, either with ‘Russet Burbank’ (seven crops) or ‘Innovator’ (four crops), the most commonly grown processing potato cultivars. The seed tubers were machine-cut to a standard weight of 100 to 150 g and treated with fungicides. Mean row width (an average of within- and between-bed width) for the 11 different crops ranged from 85 to 92 cm, plant spacing from 25 to 33 cm. Plant population, therefore, also ranged from a target population of 31,800 plants ha−1 to 45,400 plants ha−1.
Since the presence of soil-borne diseases has been strongly linked to potato cropping history (Holmstrom and Carter 2000; Larkin and Honeycutt 2006; Sinton et al. 2020), the 11 crops were selected and categorized as having been grown in fields with either no previous potato cropping history (five of the crops; designated ‘New ground’), or where potatoes had been grown within the last 7 years (six crops; ‘Old ground’). Hence the (unbalanced) survey treatments consisted of 2 cultivars and 2 histories.
Set-up
A single eight row by 10 m observation plot was set up in an area of each crop at plant emergence where population and growing conditions were representative of the field. The plot site was chosen to avoid humps, hollows, spraylines and edges. The plots were visited at 2-week intervals, until the crop canopy senescence stage for each crop (12 to 15 visits per crop). Each observation plot was systematically divided into two sections, one for crop growth measurements and concurrent disease assessments (eight plants, 2.4 m2 at three growth stages), and the other for final harvest (3 m x two rows; 4.8 m2). Crop growth rate was determined by measuring shoot and tuber biomass (at canopy closure, full canopy, and midway between full canopy and crop senescence). Final marketable tuber yield (‘plot yield’) was measured at the time of field harvest.
In-Season Measurements
At each visit, 16 plants, four from outside each corner of the observation plot, were removed and inspected for disease or physiological defects. From mid-canopy growth onwards, on three to five occasions, underground stems from these 16 plants were washed free of soil, graded for Rhizoctonia stem canker incidence and severity, and classified as healthy, diseased or dead. Presence or absence of Spongospora root galls (caused by S. subterranea) were recorded, and tubers were assessed for any surface diseases (incidence and severity) and physiological disorders. Assays for virus infections were carried out once (soon after emergence to avoid aphid vectoring) in each of the 11 crops using the methods described in Fletcher (2012). Viruses tested for were PVX = Potato X potexvirus, PVY = Potato Y potyvirus, PVS = Potato S calavirus, PVM = Potato M calavirus, PVA = Potato A potyvirus, PLRV = Potato leafroll luteovirus. Measurements of radiation interception and crop canopy cover were taken in each plot, using a handheld multispectral radiometer (CropScan Inc.; model MSR16R).
As the crops matured, six individual plants or groups of six plants showing poor vigor were identified around the perimeter of the observation plots. Symptoms were recorded, and the plants were marked for later yield and disease assessments (‘plant yield’) at harvest time. A similar number of nearby healthy plants or areas were also marked, to provide comparisons. This allowed an estimate of the likely yield if such plants comprised the entire crop. Just before the crop senescence stage (so that disease status could be confirmed), tubers from each marked plant or area were harvested, weighed and counted. Yield equivalents (t ha−1) for these plants or groups were calculated, using the per hectare plant population in each field. As a reality check, the marketable yields (‘field yield’) for the whole crops were provided by the respective farmers. This was measured by weighing the entire crop and dividing by the area harvested (a census).
At crop senescence, root distribution (subjectively characterized and scored as; 1 = very poor to 5 = excellent) was recorded in each observation plot by digging two pits excavated at right angles across a ridge and two furrows. Potato root penetration can be reduced to half its potential rate once resistance becomes >1.5 MPa, and to one quarter of the potential rate at 2.4 MPa (Stalham et al. 2007). The depths of any root restriction pans were noted and soil penetration resistance in the ridges and furrows were measured at five points within each zone with an Eijkelkamp cone penetrometer. Readings were later normalized to a fixed soil moisture content (35% [w/w] soil moisture), based on soil texture and the field soil moisture when measurements were recorded.
Analysis of Results
Potato Calculator simulations (Jamieson et al. 2003) for each crop used weather data collected at National Institute for Water and Atmospheric Research (NIWA) official weather stations at Ashburton and Timaru airports (Sinton et al. 2020). These were within one and 15 km from the individual experiment sites. The simulations were set so that water and N-fertilizer were adequate and used actual (rather than optimal) planting dates to produce time-courses of leaf area, biomass accumulation, soil moisture and soil N status. The simulated yields were compared with observed yields to assess departures of observations from predictions. The “yield gap” was defined as the difference between the observed and simulated yields predicted for production unconstrained by shortages of water or N, or by the presence of weeds, pests or diseases, and used the same cultivars and soil descriptions as a previously reported study (Sinton et al. 2020). Note model outputs are deterministic and therefore there is no method for associating uncertainty measures such as standard errors with them.
Potato Calculator estimates of potential yields using weather data from the ten previous growing seasons were used to quantify likely variations. For comparisons with grower-measured field yields, potential yields were reduced by the proportions of small tubers (<67 mm; these are rejected by the processor) measured at each site.
All growers used similar nutrient supply for their crops, with a fertilizer application (at planting) averaging 100 kg ha−1 N, 124 kg ha−1 P, 300 kg ha−1 K, 157 kg ha−1 S and a range of micronutrients. Up to a further 230 kg ha−1 N was applied as side dressings during crop growth. All the crops were irrigated with overhead sprinkler systems. However, detailed irrigation records were available only for six of the crops, and for these, the average total irrigation applied was 310 mm.
The average number of in-season fungicide applications in the 11 crops was ten (range seven to 13). Fungicides were applied to the seed potatoes and in-furrow at planting to target soil-borne diseases. The foliar fungicides applied targeted early blight and late blight. Broad spectrum strobilurin and systemic fungicides were applied early in the crop growth periods, which were likely also to provide control of other diseases (e.g. white mold). The majority of fungicide applications included two (and sometimes three), active ingredients, to reduce the risk of fungicide resistance in pathogens. The active ingredients mancozeb and chlorothalonil predominated.
The insecticides used early in the growing season (thiamethoxam, pymetrozine, spirotetramat, spiromesifen) were likely to have the least possible effects on beneficial invertebrates. Methamidophos, which harms beneficial insects, was applied only at the end of the growing season. Insecticides were applied to control aphids vectoring Potato virus Y (PVY), and to control the tomato potato psyllid (Bactericera cockerelli) which can reduce tuber yields through feeding damage and also affect tuber quality by vectoring Candidatus Liberibacter solanacearum (the cause of zebra chip in processed potato products).
Detailed Case Studies
Three growers were testing the effectiveness of remote sensing techniques as an aid to crop management (pre-plant electrical conductivity EC; remote IR thermometry from an unmanned aerial vehicle, UAV). At the planning stage, to supplement the value of their testing, we offered to make detailed growth and environmental measurements, to develop further our hypotheses from the survey year. The chosen crops were from farms with different histories and soil characteristics. One (A) had a history of short intervals between potato crops and increasing issues with soil-borne disease. Another (B) had compacted soils and limited access to water. The third (C) had unlimited water supply and deep mostly uncompacted soils. The fields all had histories of annual cropping, with two (A and C) with potato crops within the last 10 years.
The crops were all irrigated with overhead sprinkler systems and grown in fields (each ~25 ha). They were monitored for diseases, water use and tuber growth at 10- to 14-day intervals through the 2014/15 growing season. Two of the crops (A and B) were planted with ‘Russet Burbank’ and the third crop (C) was planted with ‘Innovator’. The seed tubers were treated with fungicides after cutting, and were planted with standard in-furrow fungicides to control soil-borne diseases. During the growing season, the crops were regularly sprayed to control common insect pests and foliar diseases. Crop nutrient requirements were calculated from a commercial soil test (to 150 mm depth), and were applied as pre-plant, at planting and in-season dressings. The fertilizer rates have been shown to be non-limiting at current yields in Canterbury, even when applied at double the growers’ current rates (Reid et al. 2016).
Because EC and soil water content (SWC) are associated (Grisso et al. 2009), EC was used as a guide for selecting observation plots for uniformity across each field. Eight observation plots (each eight rows by 10 m) per field were selected to represent the most common EC condition. For Fields A and C, EC to 600 mm depth was measured in transects of 15 m width, effectively measuring EC at high density across the entire field. For Field B, EC was measured to 600 mm depth in 12 m wide transects. EC values indicate differences in soil texture, cation exchange capacity, drainage conditions, organic matter content and topsoil depths (Grisso et al. 2009). At these eight plots in each crop (distributed at approximately 100 m intervals across the field), measurements of disease incidence and severity were made at 2-week intervals. On the same day, soil water content (using a neutron probe at one tube per plot) was also measured at 0.2 m increments to 0.8 m depth. Irrigation and rainfall were logged (at 1 h intervals) using automated rain gauges at three sites in each field.
From the start of tuber bulking (~60 days after crop emergence), until crop senescence, tuber yield assessments were carried out at 2-week intervals in each observation plot. For each assessment, the tubers in a 3 m by one row area were harvested, counted and weighed. Measured tuber yields were compared with values from the Potato Calculator. Canopy growth was not measured.
At each crop monitoring assessment, four pre-determined plants from each of the eight observation plots were harvested. The plants were removed to a field laboratory, and foliar diseases were recorded, and the underground stems, roots and tubers were washed and assessed for incidence and severity of diseases. Each stem was assessed for Rhizoctonia stem canker severity using a 0 to 18 scale (Michel et al. 2018). These scores reflect severity and types of lesions. Incidence of stem canker was assessed by counting the numbers of stems affected by the disease. Severity of Spongospora root galling on underground stems and roots was scored as: 1 = <5 galls/plant; 2 = 5–20 galls, or 3 = >20 galls/plant.
Mid-season, in each observation plot, a soil pit was excavated to the depth of the subsoil zone. The topsoil depth was measured and the presence of any compaction zones was assessed visually (Sinton et al. 2013).
Yield gap for each crop was quantified by comparing Potato Calculator predicted yield with crop field yield (supplied by the farmer) and plot yield for each crop. All of these yields included undersized tubers, to be consistent with the sequential measurements made during the growing season.
Statistical Analysis
Summary statistics including the mean and the standard error across the 11 survey sites (2012/2013) were calculated. Investigation of the data indicated that the degree of disease (Rhizoctonia stem canker and Spongospora root galls) observed in the plot, together with the degree of soil compaction, was related to the final yield. Four post-hoc groups in a factorial arrangement (high/low Rhizoctonia; with/without Spongospora + Compaction) were developed and tested via Analysis of Variance. We present estimated means with an associated measure of the pooled variation in the form of the 5% Least Significant Difference (LSD). For each of the 2014/2015 case studies a simple linear regression with groups (where plot was the grouping term) was fitted through time to assess whether plot to plot variation in yield remained consistent (e.g. whether plot 1 remained higher that plot 2 throughout crop development). Simple linear regression was used to fit a model to the relationship between EC and SWC for each case-study. All statistical analyses were carried out in GenStat v. 18.
Results
Potato Crop Survey
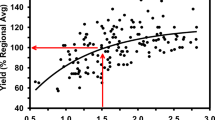
For the 11 crops monitored in the 2012/13 growing season, the overall mean final plot yield was 65 t ha−1, with a range from 53 to 79 t ha−1 (Table 1). This reflected the variability of localized yield-limiting factors. Overall mean field yields (farmer supplied) were less at 56 t ha−1, owing to a wide range of losses encountered over large crop areas, and ranged from 49 to 66 t ha−1. This variability did not relate to cultivar (mean of 58 t ha−1 for ‘Innovator’ compared with 55 t ha−1 for ‘Russet Burbank’) or cropping history (mean of 56 t ha−1 for New ground compared with 53 t ha−1 for Old ground). The mean yield gap was, therefore, 31 t ha−1 on the field scale, and 20 t ha−1 on the plot scale. Only two crops (Crops 2 and 3) came to within 10% of their respective potential yields (as indicated by the Potato Calculator), with the greatest yield gap occurring for Crop 1 (44% less than potential yield).
From the mid-canopy growth stage (approximately 100 days after planting) to senescence, the proportions of healthy, diseased and dead stems recorded from the observation plots varied between crops, and Rhizoctonia stem canker and Spongospora root galling were by far the most common stem and root diseases observed. More than 95% of assessed stems from crops 8, 9 and 10 (‘Russet Burbank’, Old Ground) were either diseased or dead during this period, with corresponding yield gaps of between 20 and 28 t ha−1 (Table 1). In contrast, Crops 2, 3, 4 (‘Innovator’) 5 and 7 (‘Russet Burbank’, New ground) had fewer diseased or dead stems (53 – 74%) and smaller yield gaps ranging from 6 to 16 t ha−1. Crops 1 and 6 diverged markedly from this trend (Fig. 1).
The scatterplot for plot yield gap versus mean Rhizoctonia stem canker incidence for 11 potato crops (numbered 1–11 as per Table 1) surveyed during the 2012/13 growing season
Rhizoctonia stem canker was found in all 11 crops, Spongospora root galling in six crops, and compacted soils occurred in six fields. Yields from the selected individual plants were categorized for the presence of soil compaction and soil-borne disease (Fig. 2). In five fields with non-compacted soils, measured yields averaged the equivalent of 92 t ha−1 for healthy plants (low Rhizoctonia stem canker severity (R), no Spongospora root galling (S), no soil compaction (C); Fig. 2). This was greater (P < 0.001) than yields of 51 t ha−1 (which was 45% less) for plants in the same fields, identified early in crop growth with severe Rhizoctonia stem canker (High R, no S, no C), leading to early plant death. With healthy plants (low stem canker severity, no root galls, compacted soils; Low R, no S, C) (four fields in this category), mean plant yield was 71 t ha−1 (23% less (P < 0.001) than Low R, no S, no C). Plants with severe stem canker, the presence of root galls, grown in compacted soils (High R, S, C) yielded the least, at 21 t ha−1 (77% less (P < 0.001) than Low R, no S, no C). Reduced weight per tuber was associated with Rhizoctonia stem canker (P < 0.001). Plants with low stem canker severities had tuber weights of 214 g and this reduced to 123 g for plants with severe stem canker.
Averaged plant yields from targeted areas in eight potato crops, categorized as having: low Rhizoctonia stem canker severity, no Spongospora root galling and no soil compaction (Low R, no S, no C); low stem canker severity, with root galls and soil compaction (Low R + S + C); high stem canker severity, no root galls and no soil compaction (High R, no S, no C); or high stem canker severity, with root galls and soil compaction both present (High R + S + C). Average LSD (0.05) 16.4 t ha−1 is indicated by the floating error-bar
Other problem areas identified in crops, from which yield losses were later measured, included poorly watered corners or outer areas of the fields (observed in two crops), extremely weedy areas (observed in two crops) and variable potato plant emergence (one crop). Lack of water reduced plant yields by the equivalent of 8 t ha−1 (62 down to 54 t ha−1) in Crop 3 and 21 t ha−1 (75 down to 54 t ha−1) in Crop 5. Weeds over-shadowing potato plants during the full canopy crop growth period caused a plant yield loss equivalent of 12 t ha−1 (89 down to 77 t ha−1) in Crop 2.
PVS (which is a mechanically transmitted virus) was at high incidence in all the crops; international research has shown yield losses from this virus can range from 4 to 20% (Fletcher 2017). PVM was detected from low to high rates in seven of the crops. Incidences of PVX, PVY, PVA and PLRV were either very low, or were not detected. However, within the scope of the survey there was no way of assessing any yield effects of PVS.
The insecticide and fungicide spray regimes used for the 11 crops adequately controlled foliar diseases, pests and insect-vectored viruses. Apart from sporadic foliar symptoms of Rhizoctonia stem canker during crop growth in most of the crops, other foliar diseases were not observed until near crop senescence. At that stage, two crops developed early blight, caused by Alternaria solani (high incidence, low severity).
When comparing fields for soil physical quality, ten fields had penetrometer resistances of less than 1.5 MPa for the within-bed furrows. However, for the wheel-track furrows, only five crops had resistances less than 1.5 MPa, while the other six crops averaged 2.4 MPa. The depth of the cultivated layers ranged from 22 to 40 cm, and six of the crops had detectable root restriction layers immediately below the cultivated layers, where roots became scarce. The majority of crops (nine) had extensive root growth within the ridges (average root distribution score = 3.9 of a maximum of 5). However, roots were less vigorous under the within-bed furrows (mean score for the 11 crops = 3.2), and less still under the wheel track furrows (mean score = 2.3). In some cases, the root distribution scores under wheel-track furrows were almost zero.
In summary, this survey established that yield losses in 11 Canterbury processing potato crops were strongly associated with the occurrence of soil-borne diseases and presence of soil compaction. All the crops developed Rhizoctonia stem canker, six of the crops had Spongospora root galling, and six crops were in compacted soils. Often occurring together, these factors were associated with inhibited plant growth and yield losses of between 20 and 42 t ha−1. Potential yields (as predicted by the Potato Calculator) were attainable in areas of the crops where plants were not affected by soil-borne diseases and had access to adequate nutrient and water resources.
Detailed Case Studies
In each of the three fields, soil electrical conductivity (EC) guided plot selection gave eight site locations where EC values were similar. When the final yields from these plots were averaged, they were within 10% of the respective final farmer-measured field yields (Table 2).
For Crop A, the average plot yield was 55 t ha−1 (range, 42 to 71 t ha−1), and the farmer field yield was 52 t ha−1. Regression analysis showed there was a weak pattern (P = 0.036) of consistency in yield variation between plots with time. The consistently greater yields later in crop growth from plots 5 and 6 (of the eight monitored plots) were probably related to increased water holding capacity of the soils in those areas. Yield accumulation had stopped at approximately 108 days after planting (DAP; Fig. 3). By 137 DAP (crop senescence), severity of Rhizoctonia stem canker had rapidly increased to reach an average score of 15.4 (Fig. 4). In Crop A the rate of development of this disease was greater than for Crops B and C, and 100% incidence of Rhizoctonia stem canker (i.e. every assessed stem) was reached by 95 DAP in Crop A. Spongospora root galling was first observed (low severity) at 82 DAP, but from 95 DAP this disease was severe until crop senescence.
For Crop B, the plot average yield was 72 t ha−1 (range, 60 to 90 t ha−1), and the farmer field yield was 66 t ha−1. Regression analysis showed there was no pattern (P = 0.27) of consistency in yield variation between plots with time, indicating that biotic factors (e.g. seed tuber quality, variability of disease) and abiotic factors (e.g. herbicide damage, localized soil physical conditions) affecting yield at each location were variable. Rhizoctonia stem canker was less severe than in Crop A, remaining below the yield limiting threshold (observed in Crop A, severity score of ≅ 5) for most of the crop growth period until 136 DAP. After this, stem canker severity increased to a score of 9 (at 157 DAP, crop senescence) (Fig. 4). In contrast with Crop A, only 65% of examined stems were affected by stem canker by 94 DAP. However, this incidence increased to 93% by crop senescence. The delayed disease development probably had limited negative effects on yield accumulation. Spongospora root galling appeared (low severity) at 123 DAP (later than at 82 DAP for Crop A), and root gall severity remained low through to crop senescence.
For Crop C, the eight-plot average yield was 78 t ha−1 (range 59 to 92 t ha−1), and farmer field yield was 70 t ha−1. The water holding capacity of the soil was greater and more homogenous across the field than for Crops A and B. Yield reduction through water shortage was very unlikely. Regression analysis showed there was a consistent pattern (P < 0.001) of yield variation with time between the eastern plots in the field (average yield = 69 t ha−1) and the western plots (average yield = 90 t ha−1). Severity of Rhizoctonia stem canker was least of the three crops studied, remaining below a mean score of 6 through to crop desiccation (158 DAP). Similar to Crop B, 65% of the stems in Crop C were affected by stem canker at 109 DAP, but numbers of infected stems increased more slowly to reach 75% affected by crop desiccation. Spongospora root galling was not observed on any plants assessed from this crop at any growth stage.
Discussion
Potato Crop Survey
Yield gaps varied substantially among crops. When all the measured yield-limiting factors were absent, plants within the crops performed to their yield potential (Fig 2). However, even when only one factor existed then plant yield was below potential. Further yield reductions were associated with additional factors. Each factor affected either root growth, root performance and/or root loss. The generated hypothesis here is that these factors caused the yield loss, because the inability of crop roots to supply necessary resources to crop canopies results in early plant senescence, and thus reduced yield.
Smaller yield gaps between plot and potential yield indicated generally good growing conditions for parts of some of the surveyed crops. In Crop 3, the low yield gap of 7 t ha−1 was associated with good soil structure and high soil organic matter content, created by 7 years of continuous grass/clover pasture prior to the potato crop. Although soil-borne diseases were present, the vigorous crop was able to produce high tuber yield. Crop 2 also had a small yield gap (6 t ha−1). Although this crop was generally overtaken by weeds (limiting field yield), the observation plot was kept weeded. Compared with the other crops in the study, the percentage of healthy stems remained high through crop growth. The soil had no compacted layer to restrict root growth.
The greatest yield gap (Crop 1, 44 t ha−1), occurred where full canopy cover was barely achieved anywhere in the crop and was not maintained for long. In addition, foliar symptoms of Rhizoctonia disease (indicating severe stem canker) became widespread. Insufficient canopy cover and premature canopy death were the likely causes of reduced tuber growth in this crop. The initial cause for the poor canopy performance in this case was unclear.
Factors other than soil-borne diseases or poor soil conditions may have contributed to the large yield gap of 34 t ha−1 for Crop 6. There was slow crop establishment, wind damage during the full canopy growth stage, and damage from the tomato potato psyllid (often vectoring Ca. L. solanacearum). These were observed in the crop, but were not quantified.
Seven of the crops had reductions in canopy duration, reducing crop yield because tubers stop growing when the canopy is no longer actively producing photosynthates. As contrasting examples, in Crop 7, the measured canopy growth and tuber yield from the monitored plot (fully irrigated) followed modelled potential, whereas in Crop 10, canopy growth was weakened by diseases, and the crop began to senesce early (Fig. 5). This was confirmed by measurements of tuber growth: tuber yield accumulation initially followed the predictions of the Potato Calculator, but then ceased early, as the canopy senesced. Canopy and tuber growth of a group of severely diseased plants within Crop 10 were even more compromised.
a Simulated and measured yield accumulation for potato Crop 7, b) canopy growth for Crop 7, c) yield accumulation for Crop 10 and d) canopy growth for Crop 10. The thick black line is the potential canopy growth for the 2012/13 season, the various dotted and dashed lines represent the 10 preceding years. The solid square symbols show canopy growth and tuber yield from the observation plot in each crop, the hollow symbols show these values for diseased plants
In the survey the plot yields were always greater than the field yields (Table 1). This was because many local areas within the crops suffered from a range of yield-limiting factors, which resulted in poor plant vigor and shortened crop canopy duration. Some of this variability was not specifically observed or measured (disease, soil water holding capacity), but other variability was explainable. For instance, some losses occurred in crop corners or edges missed by irrigation (13 and 28% yield loss in the identified inadequately irrigated areas in two crops). In Crop 7, an irrigator malfunction (three irrigator spans not delivering water) resulted in a field yield of 58 t ha−1, compared with 78 t ha−1 measured in the observation plot (in a fully irrigated zone). Losses were also associated with competition from Solanum spp. weeds (15% yield loss measured in some areas within two crops).
Apart from the spatial variations in irrigation in some of the surveyed crops, irrigation was generally adequate, so poor irrigation was unlikely to have been a widespread yield-limiting factor. The ability of the crops to access soil water was possibly an issue; results showed that the soil under implement wheel tracks was compacted, preventing potato plant root penetration (Stalham et al. 2007). At less than 1 MPa, soil compaction in the crop ridges was not limiting root growth in any of the crops. Wheel track lines are also where excess irrigation water and rainfall often ponds, removing proportions of side-dressed nitrogen through leaching. Unless crop roots were able to proliferate in other places where there was sufficient water, then overall crop growth could be reduced where soils are compacted. Deficiencies of major soil nutrients can also be ruled out as affecting yields, because doubling the normal fertilizer applications had little effect on crop yields (Reid et al. 2016).
Detailed Case Studies
The three crops behaved differently, and the size and associations of the yield gaps also differed. Crop A received adequate management to achieve maximum yield, but was overtaken by severe soil-borne diseases. Crop B had low amounts of soil-borne diseases that were unlikely to affect yield, but the yield gap was associated with inadequate water management. In Crop C, management did not restrict crop growth, and disease severity was low. The yield of this crop was closer to the potential yield than those from Crops A and B.
In Crop A, none of the monitored plots reached near to the potential yield of 102 t ha−1, even though modelling showed that water supply (rainfall plus irrigation) matched that required to achieve full yield potential (Fig. 6a). Sequential yield data showed that, compared with potential, measured tuber yield accumulation began to decrease once severity of Rhizoctonia stem canker had reached a score of approximately 5, and Spongospora root galling had become severe. Comparison of Potato Calculator estimates with measurements of soil water showed that Crop A ceased using water approximately 100 DAP (Fig. 6b), when the mean Rhizoctonia stem canker severity score was 8, and no further yield increases were measured through to crop senescence. Until then there was close agreement between measurements and simulations. Widespread and severe Rhizoctonia stem canker and Spongospora root galling (from seed tuber- and/or soil-borne inoculum) were probably the overriding factors associated with yield reduction in this crop.
a Potato Crop A, accumulated grower-applied irrigation (mm, black line) and accumulated amount predicted (mm, grey line) by the Potato Calculator required to reach potential yield; b measured soil water (mm, mean of eight plots) to 800 mm depth (black symbols) and modelled daily soil water content (using farmer-elected irrigation rate) required to achieve potential yield (grey line)
For Crop B, one of the measured areas within the crop reached near to the potential yield of 102 t ha−1, and measured tuber growth aligned with modelled yield for a longer period than that for Crop A. The amounts of disease measured were unlikely to have affected crop growth. However, sequential yield data showed a gradual reduction of tuber yield accumulation from approximately 108 DAP through to crop senescence. The Potato Calculator predicted more frequent irrigation applications were required at the individual application amount available (13 mm), but this was limited by a regional water shortage (Fig. 7a and b). Additionally, a compacted layer in the soil at 250 mm was observed to impede root growth, which probably limited water uptake by the potato plants.
a Potato Crop B, accumulated grower-applied irrigation (mm, black line) and accumulated amount predicted (mm, grey line) by the Potato Calculator required to reach potential yield; b) measured soil water (mm, mean of eight plots) to 800 mm depth (black symbols) and modelled daily soil water content (mm, using grower-elected irrigation amount) needed to achieve potential yield (mm, grey line)
Crop C was grown in a field originally divided in two, each with differing previous crop sequences. In the western segment, four measured areas of the crop reached the Potato Calculator potential of 94 t ha−1. Those in the eastern segment averaged 69 t ha−1. Measurements showed that water supply was not limiting and soil-borne disease severity was low. Previous cropping histories in the two parts of the field, the presence of a shelter belt (west), and waterlogging (east) may have affected the overall crop yields in the two field segments.
Farmers already understand that reducing the decline of soil structural quality is very important for maintaining financially viable yields in all annual crops. This is especially so for potatoes, which are recognized as weak-rooting plants. Although there is strong competition for land from use types (e.g., for milk production from pasture-grazing cows), there may be opportunity to reduce the risk of yield loss by giving consideration to selecting fields with well-structured soils. A yield-gap study conducted in 2016/17 has indicated this could be an effective strategy for reducing yield losses, by encouraging root development and plants that are resilient to disease (Sinton et al. 2020).
Conclusions
There were two objectives of this report. The major objective was to generate hypotheses about the probable cause of yield declines in potato crops. The other objective was to demonstrate the value of a community-led research effort involving collaboration among producers, processors and researchers.
From the results and discussion above, the working hypotheses for further investigation were:
-
Root and stem damage from the soil-borne diseases Spongospora root galls and Rhizoctonia stem canker cause yield decline in potato crops, which increases with increasing disease incidence and severity.
-
Yield reduction is caused by degraded soil structure and compacted zones reducing root penetration and thus access to resources. Plants in such conditions are more susceptible to disease. These soil conditions are likely to be widespread within fields and therefore, if present, will have a major effect on field yield.
-
Yield reduction is further compounded by having more than one of these yield-reducing factors present.
Both of these adverse soil conditions are likely to be associated with the length of rotation between potato crops because short rotations will support a large crop-specific pathogen population and intensive cultivation causes soil degradation. So, subsequent experimental research required treatments that would have combinations of these factors. Hence the research that followed (Sinton et al. 2020) used 4-plot layouts in each of 15 fields with differing cropping histories. The interaction between these factors was measured by using the same two cultivars reported here, grouped into four categories related to cropping history: soil-restorative (>7 years of grass) and non-restorative (>6 annual crops) with presence or absence of potato crops in the previous 10 years. A major finding was that good soil structure within the potential root zone could compensate the effects of soil-borne disease infection, to the extent that soil restorative treatment out-yielded non-restorative treatments by 10 t ha−1. The hypotheses developed in this study and tested in the subsequent study were supported.
As a result of this study and its sequel, Canterbury potato growers now pay much more attention to the history of the land chosen for potato production. Seed cutting and handling processes to limit the spread of disease have been substantially improved. At the time of submission of this paper the original report for the project (Sinton et al. 2013) had been downloaded 960 times from the Potatoes NZ website.
References
Carter, M.R., and J.B. Sanderson. 2001. Influence of conservation tillage and rotation length on potato productivity, tuber disease and soil quality parameters on a fine sandy loam in eastern Canada 2001. Soil and Tillage Research 63: 1–13.
CIP, 2018. Potato facts and figures. Available at https://cipotato.org/crops/potato/potato-facts-and-figures/. Accessed 6 Nov 2018.
Falloon, R.E., U. Merz, R.C. Butler, D. Curtin, R.A. Lister, and S.M. Thomas. 2016. Root infection of potato by Spongospora subterranea: Knowledge review and evidence for decreased plant productivity. Plant Pathology 65: 422–434.
FAO, 2008. Sustainable potato production. Guidelines for developing countries. www.fao.org/3/a-i1127e.pdf. Accessed 6 November 2018.
Fletcher, J.D. 2012. A virus survey of New Zealand fresh, process and seed potato crops during 2010–11. New Zealand Plant Protection 65: 197–203.
Fletcher, J.D., 2017. How important is Potato virus S (PVS)? Potatoes New Zealand archive. https://potatoesnz.co.nz/updates/biosecurity-updates/important-potato-virus-s-pvs/. Accessed 6 Nov 2018.
Gebru, H., A. Mohammed, N. Dechassa, and D. Belew. 2017. Assessment of production practices of smallholder potato (Solanum tuberosum L.) farmers in Wolaita zone, southern Ethiopia. Agriculture & Food Security 6: 1–11.
Grisso, R., M. Alley, D. Holshouser, W. Thomason, 2009. Precision farming tools: soil electrical conductivity. Virginia Cooperative Extension. http://pubs.ext.vt.edu/442/442-508/442-508.html. Accessed 6 Nov 2018.
Harahagazwe, D., B. Condori, C. Barreda, A. Bararyenya, A.A. Byarugaba, D.A. Kude, C. Lung'aho, C. Martinho, D. Mbiri, B. Nasona, B. Ochieng, J. Onditi, J.M. Randrianaivoarivony, C.M. Tankou, A. Worku, E. Schulte-Geldermann, V. Mares, F. de Mendiburu, and R. Quiroz. 2018. How big is the potato (Solanum tuberosum L.) yield gap in sub-Saharan Africa and why? A participatory approach. Open Agriculture 3: 180–189.
Haverkort, A.J., and P.C. Struik. 2015. Yield levels of potato crops: Recent achievements and future prospects. Field Crops Research 182: 76–85.
Holmstrom, D.A., and M.R. Carter. 2000. Effect of subsoil tillage in the previous crop year on soil loosening and potato yield performance. Canadian Journal of Plant Science 80: 161–164.
Jamieson, P.D., 1985. Irrigation response of potatoes. In “Potato growing – a changing scene.” NZ Agronomy Society Special Publication 3: 17-20.
Jamieson, P.D., P.J. Stone, R.F. Zyskowski, and S. Sinton. 2003. Implementation and testing of the potato calculator, a decision support system for nitrogen and irrigation management. In Decision support systems in potato production: Bringing models to practice, ed. A.J. Haverkort and D.K.L. Mackerron, 85–99. Wageningen Academic Publishers.
Jamieson, P.D., R.F. Zyskowski, S.M. Sinton, H.E. Brown, and R.C. Butler. 2006. The potato calculator: A tool for scheduling nitrogen fertilizer applications. Agronomy New Zealand 36: 49–53.
Jamieson, P.D., R.F. Zyskowski, F.Y. Li, and M.A. Semenov. 2008. Water and nitrogen uptake and responses in models of wheat, potatoes and maize. In Quantifying and understanding plant nitrogen uptake for systems modelling, ed. L. Ma, L.R. Ahuja, and T.W. Bruulsema, 127–145. Abingdon, Oxon, UK: CRC Press.
Larkin, R.P., and C.W. Honeycutt. 2006. Effects of different 3-year cropping systems on soil microbial communities and rhizoctonia diseases of potato. Phytopathology 96: 68–79. https://doi.org/10.1094/PHYTO-96-0068.
Martin, R.J., P.D. Jamieson, D.R. Wilson, and G.S. Francis. 1991. Effects of soil moisture deficits on yield and quality of ‘russet Burbank’ potatoes. New Zealand Journal of Crop and Horticultural Science 20: 1–9.
McCulloch J., S. Sinton, F Shah, K. Richards, 2018. The effects of formaldehyde potato seed treatment on plant health and yield. https://potatoesnz.co.nz/wp-content/uploads/2018/03/RDE-The-Effects-Of-Formaldehyde-Potato-Seed-Treatment-FINAL-McCulloch-J-et-al-20180831.pdf. Accessed 22 June 2020.
Michel A, Brown H, Sinton S, Meenken E, Dellow S, Searle B, Reid J. 2013. Potato Yield Gap investigation 2012–13. Part B: Effect of nutrient supply on yield. Plant & Food Research confidential report no. 8620. https://potatoesnz.co.nz/mdocs-posts/pfr8620-potato-yield-gap-investigation-2012-13-part-b-effect-of-nutrient-supply-on-yield/. Accessed 14 Aug 2021.
Michel, A.J., R.E. Falloon, K.K. Richards, S.M. Sinton, F.A. Shah, and S.J. Dellow. 2018. Methods for measuring Rhizoctonia stem canker severity in potato crops. Agronomy New Zealand 48: 165–176.
Monteith, J.L. 1977. Climate and the efficiency of crop production in Britain. Philosophical Transactions of the Royal Society of London B281: 277–294.
Reid, J.B., B.P. Searle, and S. Sinton. 2011. Does my potato crop need fertiliser? A modelling approach Agronomy New Zealand 41: 79–95.
Reid, J.B., B. Searle, S. Sinton, A. Michel, E. Meenken, H. Brown, A. Roberts, and M. Manning. 2016. Fertiliser practice and yield losses in process potato crops grown in Canterbury, New Zealand. New Zealand Journal of Crop and Horticultural Science 44: 1–17.
Roberto, Q.R., D. Harahagazwe, B. Condori, C. Barreda, F. Mendiburu, A. Amele, 2014. Potato yield gap analysis in SSA through participatory modeling: Optimizing the value of historical breeding trial data. CIP working paper. http://humidtropics.cgiar.org/wp-content/uploads/downloads/2014/04/Potato-Yield-Gap-Analysis.pdf. Accessed 22 Nov 2018.
Sinton S, H. Brown, E. Meenken, 2009. Nitrogen management of North Island potato crops, 2005–08. Plant & Food Research Confidential Report no. 2321. https://potatoesnz.co.nz/rd-project/the-potato-calculator/. Accessed 5 Oct 2021.
Sinton S., R. Falloon, H. Brown, C. Tregurtha, A. Michel, S. Dellow, J. Reid, F. Shah, S. Pethybridge, B. Searle, 2013. Potato yield gap investigation 2012-13. Part A, factors limiting yield. SPTS No 8706. https://potatoesnz.co.nz/mdocs-posts/0136-8706-potato-yield-gap-investigation-2012-13-part-a-factors-limiting-yield-1. Accessed 14 Aug 2021.
Sinton, S.M., S. Dellow, P. Jamieson, R. Falloon, F. Shah, E. Meenken, K. Richards, A. Michel, C. Tregurtha, and J. McCulloch. 2020. Cropping history affects potato yields in Canterbury, New Zealand. American Journal of Potato Research American Journal of Potato Research 97: 202–213.
Stalham, M., E. Allen, A. Rosenfeld, and F. Herry. 2007. Effects of soil compaction in potato (Solanum tuberosum) crops. The Journal of Agricultural Science 145: 295–312.
Tolno, E., H. Kobayashi, M. Ichizen, M. Esham, and B. Siddighi. 2016. Potato production and supply by smallholder farmers in Guinea: An economic analysis. Asian Journal of Agricultural Extension, Economics & Sociology 8: 1–16. https://doi.org/10.9734/AJAEES/2016/21726.
Acknowledgements
The project team thanks Canterbury farmers for allowing access to their fields and crop management information, and also acknowledges the assistance of Plant & Food Research colleagues and local agronomists for the sharing of their expertise during this study. This research was jointly funded by Potatoes New Zealand, McCain Foods Ltd. Grower Group, Ravensdown Fertiliser Co-operative Limited and Plant & Food Research. This research is part of the Plant & Food Research ‘Profitable and Sustainable Potato Production Systems’ Programme, funded by the New Zealand Ministry of Business, Innovation and Employment (MBIE) through the Strategic Science Investment Fund (SSIF).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors affirm that none has a conflict of interest associated with any statement made, products mentioned, or conclusions drawn in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sinton, S.M., Falloon, R.E., Jamieson, P.D. et al. Yield Depression in New Zealand Potato Crops Associated with Soil Compaction and Soil-Borne Diseases. Am. J. Potato Res. 99, 160–173 (2022). https://doi.org/10.1007/s12230-022-09864-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-022-09864-5