Abstract
Purpose of Review
Echocardiography is an integral component in the diagnosis and management of heart disease. This review focuses on the applications of artificial intelligence in echocardiography.
Recent Findings
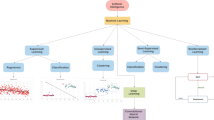
Artificial intelligence (AI) and machine learning (ML) applications continue to prove as useful adjuncts in clinical echocardiography. AI has proven useful in echocardiographic assessment of chamber size and function and Doppler measurement.
Similarly, AI applications have demonstrated benefit in the echocardiographic diagnosis of valvular disease and cardiomyopathies as well as predicting outcomes and prognostication. Additionally, recent studies have shown the value of AI in acquisition of limited echocardiographic views with the ability to assist in the training of novices to acquire these images quickly.
Summary
AI is a valuable adjunct in clinical echocardiography with the potential for improving diagnostic efficiency and accuracy, reducing costs, and personalizing cardiac care.



Reproduced with permission from Nedadur et al. Artificial intelligence for the echocardiographic assessment of valvular heart disease. Heart. 2022 Feb 10:heartjnl-2021–319,725.

Similar content being viewed by others
References
Szolovits P, Patil RS, Schwartz WB. Artificial intelligence in medical diagnosis. Ann Intern Med. 1988;108:80–7.
Dey D, Slomka PJ, Leeson P, et al. Artificial intelligence in cardiovascular imaging: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1317–35.
Kusunose K, Haga A, Abe T, Sata M. Utilization of artificial intelligence in echocardiography. Circ J. 2019;83:1623–9.
Deo RC. Machine learning in medicine. Circ. 2015;132:1920–30.
LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521:436–44.
Zhang J, Gajjala S, Agrawal P, et al. Fully automated echocardiogram interpretation in clinical practice. Circ. 2018;138:1623–35.
Narang A, Bae R, Hong H, et al. Utility of a deep-learning algorithm to guide novices to acquire echocardiograms for limited diagnostic use. JAMA Cardiol. 2021;6:624–32.
Abdi AH, Luong C, Tsang T, et al. Automatic quality assessment of echocardiograms using convolutional neural networks: feasibility on the apical four-chamber view. IEEE Trans Med Imaging. 2017;36:1221–30.
Khamis H, Zurakhov G, Azar V, Raz A, Friedman Z, Adam D. Automatic apical view classification of echocardiograms using a discriminative learning dictionary. Med Image Anal. 2017;36:15–21.
Madani A, Arnaout R, Mofrad M, Arnaout R. Fast and accurate view classification of echocardiograms using deep learning. NPJ Digit Med. 2018;1:6. https://doi.org/10.1038/s41746-017-0013-1.
Kusunose K, Haga A, Inoue M, Fukuda D, Yamada H, Sata M. Clinically feasible and accurate view classification of echocardiographic images using deep learning. biomolecules. 2020;10(5):665. https://doi.org/10.3390/biom10050665.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1–39):e14.
Tsang W, Salgo IS, Medvedofsky D, et al. Transthoracic 3D echocardiographic left heart chamber quantification using an automated adaptive analytics algorithm. JACC Cardiovasc Imaging. 2016;9:769–82.
Medvedofsky D, Mor-Avi V, Amzulescu M, et al. Three-dimensional echocardiographic quantification of the left-heart chambers using an automated adaptive analytics algorithm: multicentre validation study. Eur Heart J Cardiovasc Imaging. 2018;19:47–58.
Tamborini G, Piazzese C, Lang RM, et al. Feasibility and accuracy of automated software for transthoracic three-dimensional left ventricular volume and function analysis: comparisons with two-dimensional echocardiography, three-dimensional transthoracic manual method, and cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2017;30:1049–58.
Levy F, Dan Schouver E, Iacuzio L, et al. Performance of new automated transthoracic three-dimensional echocardiographic software for left ventricular volumes and function assessment in routine clinical practice: comparison with 3 Tesla cardiac magnetic resonance. Arch Cardiovasc Dis. 2017;110:580–9.
Sun L, Feng H, Ni L, Wang H, Gao D. Realization of fully automated quantification of left ventricular volumes and systolic function using transthoracic 3D echocardiography. Cardiovasc Ultrasound. 2018;16:2.
Medvedofsky D, Mor-Avi V, Byku I, et al. Three-dimensional echocardiographic automated quantification of left heart chamber volumes using an adaptive analytics algorithm: feasibility and impact of image quality in nonselected patients. J Am Soc Echocardiogr. 2017;30:879–85.
Narang A, Mor-Avi V, Prado A, et al. Machine learning based automated dynamic quantification of left heart chamber volumes. Eur Heart J Cardiovasc Imaging. 2019;20:541–9.
D’Elia N, Appadurai V, Mallouhi M, Ng J, Marwick T, Wahi S. Comparison of 3D echocardiographic-derived indices using fully automatic left ventricular endocardial tracing (heart model) and semiautomatic tracing (3DQ-ADV). Echocardiography. 2019;36:2057–63.
Volpato V, Mor-Avi V, Narang A, et al. Automated, machine learning-based, 3D echocardiographic quantification of left ventricular mass. Echocardiogr. 2019;36:312–9.
Barbieri A, Bursi F, Camaioni G, Maisano A, Imberti JF, Albini A, De Mitri G, Mantovani F, Boriani G. Echocardiographic left ventricular mass assessment: correlation between 2D-derived linear dimensions and 3-Dimensional automated, machine learning-based methods in unselected patients. J Clin Med. 2021;10(6):1279. https://doi.org/10.3390/jcm10061279.
Qazi M, Fung G, Krishnan S, Bi J, Rao RB, Katz AS. Automated heart abnormality detection using sparse linear classifiers. IEEE Eng Med Biol Mag. 2007;26:56–63.
Huang MS, Wang CS, Chiang JH, Liu PY, Tsai WC. Automated recognition of regional wall motion abnormalities through deep neural network interpretation of transthoracic echocardiography. Circ. 2020;142:1510–20.
Lin X, Yang F, Chen Y, et al. Echocardiography-based AI detection of regional wall motion abnormalities and quantification of cardiac function in myocardial infarction. Front Cardiovasc Med. 2022;9:903660.
Gosling AF, Thalappillil R, Ortoleva J, Datta P, Cobey FC. Automated spectral Doppler profile tracing. J Cardiothorac Vasc Anesth. 2020;34:72–6.
Nolan MT, Thavendiranathan P. Automated quantification in echocardiography. JACC Cardiovasc Imaging. 2019;12:1073–92.
Moghaddasi H, Nourian S. Automatic assessment of mitral regurgitation severity based on extensive textural features on 2D echocardiography videos. Comput Biol Med. 2016;73:47–55.
Martins J, Nascimento ER, Nascimento BR, et al. Towards automatic diagnosis of rheumatic heart disease on echocardiographic exams through video-based deep learning. J Am Med Inform Assoc. 2021;28:1834–42.
Vafaeezadeh M, Behnam H, Hosseinsabet A, Gifani P. A deep learning approach for the automatic recognition of prosthetic mitral valve in echocardiographic images. Comput Biol Med. 2021;133:104388.
Aquila I, Fernandez-Golfin C, Rincon LM, et al. Fully automated software for mitral annulus evaluation in chronic mitral regurgitation by 3-dimensional transesophageal echocardiography. Med. 2016;95:e5387.
Calleja A, Thavendiranathan P, Ionasec RI, et al. Automated quantitative 3-dimensional modeling of the aortic valve and root by 3-dimensional transesophageal echocardiography in normals, aortic regurgitation, and aortic stenosis: comparison to computed tomography in normals and clinical implications. Circ Cardiovasc Imaging. 2013;6:99–108.
Garcia-Martin A, Lazaro-Rivera C, Fernandez-Golfin C, et al. Accuracy and reproducibility of novel echocardiographic three-dimensional automated software for the assessment of the aortic root in candidates for thanscatheter aortic valve replacement. Eur Heart J Cardiovasc Imaging. 2016;17:772–8.
Narula S, Shameer K, Salem Omar AM, Dudley JT, Sengupta PP. Machine-learning algorithms to automate morphological and functional assessments in 2D echocardiography. J Am Coll Cardiol. 2016;68:2287–95.
Sengupta PP, Huang YM, Bansal M, Ashrafi A, Fisher M, Shameer K, Gall W, Dudley JT. Cognitive machine-learning algorithm for cardiac imaging: a pilot study for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ Cardiovasc Imaging. 2016;9(6):e004330. https://doi.org/10.1161/CIRCIMAGING.115.004330.
Berchialla P, Foltran F, Bigi R, Gregori D. Integrating stress-related ventricular functional and angiographic data in preventive cardiology: a unified approach implementing a Bayesian network. J Eval Clin Pract. 2012;18:637–43.
Ernande L, Audureau E, Jellis CL, et al. Clinical implications of echocardiographic phenotypes of patients with diabetes mellitus. J Am Coll Cardiol. 2017;70:1704–16.
Agasthi P, Buras MR, Smith SD, et al. Machine learning helps predict long-term mortality and graft failure in patients undergoing heart transplant. Gen Thorac Cardiovasc Surg. 2020;68:1369–76.
Nabi W, Bansal A, Xu B. Applications of artificial intelligence and machine learning approaches in echocardiography. Echocardiogr. 2021;38:982–92.
Krittanawong C, Johnson KW, Rosenson RS, et al. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J. 2019;40:2058–73.
Olson RS, Cava W, Mustahsan Z, Varik A, Moore JH. Data-driven advice for applying machine learning to bioinformatics problems. Pac Symp Biocomput. 2018;23:192–203.
Funding
AN is the recipient of funding from the Brenda and Lance Feis Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Slostad, B., Karnik, A., Appadurai, V. et al. Applications of Artificial Intelligence in Echocardiography. Curr Cardiovasc Risk Rep 17, 123–132 (2023). https://doi.org/10.1007/s12170-023-00721-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12170-023-00721-6