Abstract
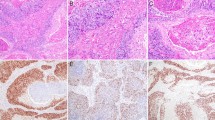
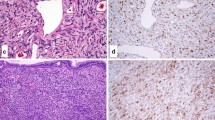
Sinonasal teratocarcinosarcoma (SNTCS) is a rare, aggressive malignancy that displays a heterogeneous combination of malignant blastema-like, epithelial and mesenchymal components. Its exact histogenesis is unknown with hypotheses ranging from true germ cell derivation to origin from pluripotent stem cells. However, despite this tumor’s multiphenotypic histology, which includes frequent glandular, squamous, and neuroectodermal differentiation similar to adnexal germ cell tumors, SNTCS appears to have some differences from adnexal teratomas. For example, unlike adnexal teratomas, SNTCS has never been described as a component in a mixed germ cell tumor. Accurate recognition of SNTCS is difficult due to its rarity and histologic overlap with other sinonasal tumors. It is even more problematic on biopsy, since not all elements may be present in small samples. SNTCS can also share similar staining patterns with other neoplasms in the differential diagnosis. A recent study found nuclear β-catenin expression in a single TCS, but this has yet to be confirmed in additional cases. SALL-4, a marker of germ cell tumors, has not been examined. We performed β-catenin and SALL-4 immunohistochemistry on whole sections of 7 SNTCS and 19 other sinonasal neoplasms to assess whether β-catenin and SALL-4 are of utility in establishing a diagnosis of SNTCS. Intensity of expression and percentage of staining was noted for each tumor. For SNTCS, distribution of staining within each histologic component (immature neuroectodermal, epithelial, and mesenchymal) was also documented. Nuclear β-catenin expression was not identified in any SNTCS, with all cases demonstrating membranous expression (6 cases) or cytoplasmic and membranous expression (1 case). SALL-4 immunohistochemistry, however, was relatively sensitive (85.7%) and specific (89.5%) for SNTCS. SALL-4 expression was also identified in one poorly differentiated neuroendocrine carcinoma and one case of sinonasal undifferentiated carcinoma. SALL-4 appears to have utility in distinguishing SNTCS from other high grade sinonasal tumors.


Similar content being viewed by others
Data Availability
Raw data will be shared upon request.
References
Dutta R, Dubal PM, Svider PF, Liu JK, Baredes S, Eloy JA. Sinonasal malignancies: a population-based analysis of site-specific incidence and survival. Laryngoscope. 2015;125(11):2491–7.
El-Naggar AK, Chan JKC, Grandis JR, Slootweg PJ. Tumors of the nasal cavity, paranasal sinuses and skull base. In: WHO Classification of Head and Neck Tumours. Lyon: International Agency for Research on Cancer; 2017. p. 26–7.
Misra P, Husain Q, Svider PF, Sanghvi S, Liu JK, Eloy JA. Management of sinonasal teratocarcinosarcoma: a systematic review. Am J Otolaryngol. 2014;35(1):5–11.
Smith SL, Hessel AC, Luna MA, et al. Sinonasal teratocarcinosarcoma of the head and neck: a report of 10 patients treated at a single institution and comparison with reported series. Arch Otolaryngol Head Neck Surg. 2008;134:592–5.
Budrukkar A, Agarwal JP, Kane S, et al. Management and clinical outcome of sinonasal teratocarcinosarcoma: single institution experience. J Laryngol Otol. 2010;124(7):739–43. https://doi.org/10.1017/S0022215109992866.
Su YS, Bell D, Hanna YE, Esthesioneuroblastoma. Neuroendocrine carcinoma, and sinonasal undifferentiated carcinoma: differentiation in diagnosis and treatment. Int Arch Otorhinolaryngol. 2014;18:149–56.
Birkeland AC, Burgin SJ, Yanik M, Scott MV, Bradford CR, McHugh JB, Brenner JC. Pathogenetic analysis of sinonasal teratocarcinosarcomas reveal actionable β-catenin overexpression and a β-catenin mutation. J Neurol Surg Part B Skull Base. 2017;78(4):346–52.
Rooper L, Nasir UD, Gagan J, Thompson L, Agaimy A, Bishop J. Comprehensive molecular profiling of sinonasal teratocarcinosarcoma identifies recurrent SMARCA4 and Wnt/β-catenin pathway mutations. USCAP 110th Annual Meeting Abstracts. Mod Pathol. 2021;34(S2):787–8.
MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. https://doi.org/10.1016/j.devcel.2009.06.016.
Brembeck FH, Rosário M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16(1):51–9.
Stanczak A, Stec R, Bodnar L, et al. Prognostic significance of Wnt-1, β-catenin and E-cadherin expression in advanced colorectal carcinoma. Pathol Oncol Res. 2011;17(4):955–63.
Schlosshauer PW, Ellenson LH, Soslow RA. Beta-catenin and E-cadherin expression patterns in high-grade endometrial carcinoma are associated with histological subtype. Mod Pathol. 2002;15(10):1032–7.
Burford H, Baloch Z, Liu X, Jhala D, Siegal GP, Jhala N. E-cadherin/beta-catenin and CD10: a limited immunohistochemical panel to distinguish pancreatic endocrine neoplasm from solid pseudopapillary neoplasm of the pancreas on endoscopic ultrasound-guided fine-needle aspirates of the pancreas. Am J Clin Pathol. 2009;132(6):831–9.
Cao D, Li J, Guo CC, Allan RW, Humphrey PA. SALL4 is a novel diagnostic marker for testicular germ cell tumors. Am J Surg Pathol 2009;33(7):1065–77.
Salem F, Rosenblum MK, Jhanwar SC, Kancherla P, Ghossein RA, Carlson DL. Teratocarcinosarcoma of the nasal cavity and paranasal sinuses: report of 3 cases with assessment for chromosome 12p status. Hum Pathol. 2008;39(4):605–9. https://doi.org/10.1016/j.humpath.2007.09.002.
Rooper LM, Uddin N, Gagan J, et al. Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol. 2020;44(10):1331–9. https://doi.org/10.1097/PAS.0000000000001508.
Chan-Penebre E, Armstrong K, Drew A, et al. Selective Killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: in vitro and in vivo preclinical models. Mol Cancer Ther. 2017;16(5):850–60. https://doi.org/10.1158/1535-7163.MCT-16-0678.
Tagal V, Wei S, Zhang W, et al. SMARCA4-inactivating mutations increase sensitivity to Aurora kinase A inhibitor VX-680 in non-small cell lung cancers. Nat Commun. 2017;8:14098. https://doi.org/10.1038/ncomms14098.
Xue Y, Meehan B, Macdonald E, et al. CDK4/6 inhibitors target SMARCA4-determined cyclin D1 deficiency in hypercalcemic small cell carcinoma of the ovary. Nat Commun. 2019;10:558.
Bell EH, Chakraborty AR, Mo X, et al. SMARCA4/BRG1 is a novel prognostic biomarker predictive of cisplatin-based chemotherapy outcomes in resected non-small cell lung cancer. Clin Cancer Res. 2016;22(10):2396–404. https://doi.org/10.1158/1078-0432.CCR-15-1468.
Fatima SS, Minhas K, Din NU, Fatima S, Ahmed A, Ahmad Z. Sinonasal teratocarcinosarcoma: a clinicopathologic and immunohistochemical study of 6 cases. Ann Diagn Pathol. 2013;17(4):313–8. https://doi.org/10.1016/j.anndiagpath.2013.01.003.
McCuiston A, Bishop JA. Usefulness of NKX2.2 immunohistochemistry for distinguishing Ewing sarcoma from other sinonasal small round blue cell tumors. Head Neck Pathol. 2018;12(1):89–94. https://doi.org/10.1007/s12105-017-0830-1.
Griffin CT, Curtis CD, Davis RB, Muthukumar V, Magnuson T. The chromatin-remodeling enzyme BRG1 modulates vascular Wnt signaling at two levels. Proc Natl Acad Sci U S A. 2011;108(6):2282–7.
Dengfeng Cao S, Guo RW, Allan, Kyle H, Molberg Y, Peng. SALL4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in distinguishing yolk sac tumor from clear cell carcinoma. Am J Surg Pathol. 2009;33(6):894–904.
Perret R, Chalabreysse L, Watson S, Serre I, et al. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cases with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol. 2019;43(4):455–65. https://doi.org/10.1097/PAS.0000000000001188.
Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. 2020;15(2):231–47. https://doi.org/10.1016/j.jtho.2019.10.023.
Kidder BL, Palmer S, Knott JG. SWI/SNF-Brg1 regulates self-renewal and occupies core pluripotency-related genes in embryonic stem cells. Stem Cells. 2009;27(2):317–28.
Xiong J, Todorova D, Su NY, et al. Stemness factor Sall4 is required for DNA damage response in embryonic stem cells. J Cell Biol. 2015;208(5):513–20.
Agaimy A, Jain D, Uddin N, Rooper LM, Bishop JA. SMARCA4-deficient sinonasal carcinoma: a series of 10 cases expanding the genetic spectrum of SWI/SNF-driven sinonasal malignancies. Am J Surg Pathol. 2020;44(5):703–10.
Funding
No external funding was received. The research was performed using internal Vanderbilt faculty funds provided by author KAE.
Author information
Authors and Affiliations
Contributions
MLC: Design, case selection, data collection and analysis, manuscript drafting, review and final approval of manuscript. JSL: Case selection, review and final approval of manuscript. WCF: Case selection, review and final approval of manuscript. NAC: Case selection, review and final approval of manuscript. QS: Case selection, review and final approval of manuscript. KAE: Design, case selection, data collection and analysis, manuscript drafting, review and final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Consent to Participate
Waiver of consent (research performed on anonymized archival materials).
Consent for Publication
Waiver of consent (research performed on anonymized archival materials).
Ethical Approval
Approved by Vanderbilt IRB board.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Compton, M.L., Lewis, J.S., Faquin, W.C. et al. SALL-4 and Beta-Catenin Expression in Sinonasal Teratocarcinosarcoma. Head and Neck Pathol 16, 229–235 (2022). https://doi.org/10.1007/s12105-021-01343-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12105-021-01343-3