Abstract
Background
Poorly differentiated sinonasal small round cell tumors (SRCTs) are rare and heterogeneous, posing challenges in diagnosis and treatment.
Methods
Recent advances in molecular findings and diagnostic refinement have promoted better understanding and management of these tumors.
Results
The newly defined and emerging sinonasal entities demonstrate diverse morphologies, specific genomic signatures, and clinical behavior from conventional counterparts. In this review of SRCTs, emphasis is placed on the diagnostic approach with the employment of a pertinent panel of immunohistochemistry studies and/or molecular tests, fine-tuned to the latest WHO 5 classification of sinonasal/paranasal tumors and personalized treatment.
Conclusion
Specifically, this review focuses on tumors with epithelial and neuroectodermal derivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variegated epithelial, neuroectodermal, mesenchymal, and hematolymphoid neoplasms arise in the sinonasal cavities, accounting for approximately 3–5% of all head and neck tumors. With a combined incidence of 0.5–1.0 cases per 100,000 per year, sinonasal malignancies are considered rare cancers. While rare tumors account for approximately 20% of all cancer patients, new advances lag behind those reported in more common solid tumors, with few clinical trials currently benefiting these patients [1, 2]. Moreover, sinonasal tumors present challenges to clinical management due to anatomic considerations, along with their distinctive etiologies, epidemiology, clinical and genetic characteristics. Nevertheless, progress has been made in recent decades regarding surgical techniques, imaging modalities, and radiotherapy and in the identification of molecular alterations that may improve diagnosis, identification of new entities, prognosis, and the stratification of treatment.
Sinonasal small round cell tumors (SRCTs) constitute a heterogeneous group of malignant neoplasms characterized by a monotonous population of undifferentiated tumor cells with a relatively high nuclear to cytoplasmic ratio and high mitotic activity in conventional H&E light microscopy. An early and accurate diagnosis is imperative so patients with paranasal/nasal cavity SRBCTs can undergo appropriate therapy. Completing a definitive diagnosis of an SRCT based solely on H&E findings may be exceedingly difficult because of the frequent absence of distinguishing features. In routine practice, additional challenges include suboptimal diagnostic tissue (small, crushed, poorly preserved, necrotic, fibrotic, predominantly blood clot) and sampling errors [3]. For an accurate diagnosis in this group of tumors, there is a heavy reliance on ancillary studies, including a broad panel of immunohistochemical stains and molecular studies [3].
In this review of SRCTs, emphasis is placed on the diagnostic approach with the employment of a pertinent panel of immunohistochemistry studies and/or molecular tests fine-tuned to the latest WHO 5 classification of sinonasal/paranasal tumors and personalized treatment.
Poorly Differentiated High-Grade Sinonasal Carcinomas
Nuclear Protein in Testis (NUT) Carcinoma (NC)
Nuclear protein in testis (NUT) carcinomas (NCs) are rare, clinically aggressive carcinomas that are characterized by a translocation involving the NUTM1 gene on chromosome 15q14 and, in most cases (~ 70–80%), the bromodomain-containing 4 (BRD4) gene on chromosome 19p13.1, resulting in a BRD4-NUTM1 fusion oncogene [4, 5]. Other variant rearrangements include the BRD3-NUTM1 fusion (~ 15–20%) [6] and NSD3-NUTM1 fusion (~ 6%) [7], among partner genes (zinc finger ZNF52, ZNF592 in ~ 2%) [8]. In a subset of malignant solid tumors from soft tissue and other organs of uncertain relationship to NCs, NUTM1 has been reported to be fused with other genes (YAP1, MXD1, MXD4, CIC, BCORL1, ATXN1, and MGA); these genes have been described to occur in high-grade sarcoma associated with a distinct pathogenetic pathway (reviewed in Moreno et al. [9]).
NCs are composed of undifferentiated basaloid cells with focal, often abrupt, squamous differentiation [10]. NCs can mimic other undifferentiated neoplasms, such as pediatric small blue cell tumors, germ cell tumors, Ewing sarcoma, lymphoma, or SNUC. NUT carcinomas have an epithelial immunophenotype and focally express keratin, p63, CK7, CK20, and CK34, which reflect varying degrees of squamous differentiation. An extensive panel of lineage immunomarkers (e.g., desmin, myoglobin, smooth muscle actin, muscle actin, chromogranin, synaptophysin, leukocyte common antigen, placental alkaline phosphatase, S100 protein, alpha fetoprotein, neuron-specific enolase, CD57, CD99, HMB45) are not expressed in NCs. Oncoviruses, such as Epstein‒Barr virus and HPV, have not been reported thus far in NCs; their presence would likely exclude this diagnosis. Demonstration of the NUT translocation is needed for definitive diagnosis of NCs; this can be achieved by karyotyping, reverse transcription polymerase chain reaction, fluorescence in situ hybridization (FISH), and next-generation sequencing (NGS)- or whole-exome sequencing (WES)-based approaches (reviewed in Moreno et al. [9]).
Immunohistochemistry for NUT represents an acceptable surrogate marker, with NCs showing a nuclear staining pattern. Immunostaining with a monoclonal antibody to NUT has a sensitivity of 87%, a specificity of 100%, a negative predictive value of 99%, and a positive predictive value of 100% in distinguishing NCs from other poorly differentiated sinonasal carcinomas [11]. Given the anecdotal favorable responses of NUTs to certain treatment regimens, including chemotherapy according to Ewing sarcoma protocols or docetaxel and radiotherapy [12, 13], the distinction of NCs from other sinonasal carcinomas appears to be of clinical relevance. Targeted therapy using small-molecule BET inhibitors has shown activity but no obvious survival benefits, most likely due to toxicity effects [14]. Any poorly differentiated midline carcinoma or head and neck tumor lacking lineage-specific differentiation markers should be considered for immunostaining for NUT or rearrangement testing.
MYC has been shown to be a downstream oncogene target of BRD4::NUTM1 that blocks NC cellular differentiation and maintains a proliferative state [15]. The transcription factor SOX2 (sex-determining region Y-box protein 2), which is essential for stem cell self-renewal and pluripotency, is also an oncogenic target of BRD4::NUTM1 [16, 17]. BRD4::NUTM1 has been shown to drive overexpression of SOX2 in NUT carcinoma cells, which induces an aberrant stem cell-like growth feature [17]. Sox2 expression is normally restricted to stem cells; its aberrant overexpression has been linked to the ability to promote tumorigenicity and a poorly differentiated morphology [18,19,20]. Sox2 expression and gene amplification have been identified as common events in the head and neck [21, 22]; in the sinonasal region, amplification and/or overexpression of Sox2 has been demonstrated in squamous carcinoma (SNSCC), sinonasal undifferentiated carcinoma (SNUC), adenoid cystic carcinoma (AdCC), and intestinal type adenocarcinoma (ITAC) [22,23,24]. Although the literature is controversial regarding SOX2 amplification/Sox2 expression, recent data highlight the driver role of SOX2 in stemness with Sox2 overexpression and poor outcomes in patients with solid tumors [25]. Sox2 expression is also associated with resistance to chemotherapy through a plethora of mechanisms, and as such is a promising target for anticancer therapy [22, 26].
Tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs) have been discovered within recent decades [27]. TSAs may result from gene mutations or from the expression of alternative open reading frames, resulting from chromosomal rearrangements; normal tissues frequently carry TAAs, with the drawback of autoimmunity development in parallel to conferring tolerance to these antigens through vaccination and tumor recognition 27,28,29]. Preferentially expressed antigen in melanoma (PRAME) is a testis-selective cancer testis antigen with restricted expression in somatic tissues and re-expression in various cancers. PRAME has gained interest as a candidate target for immunotherapy [30]. PRAME plays a role in the acquisition of various cancer hallmarks, including replicative immortality or stemness, invasion, and metastasis [30]. In addition to supporting tumor features, PRAME has been implicated in the regulation of the immune response [31].
In a recent study that aimed to characterize the immune-oncology gene expression profile in sinonasal undifferentiated carcinomas (SNUCs) and other high-grade sinonasal carcinomas, PRAME was the top upregulated gene in SNUCs and SWI/SNF-deficient sinonasal carcinomas (fold change 8.40), and fold change half values (4.8) were observed for high-grade neuroendocrine carcinomas (HGNECs) [32]. PRAME protein overexpression has also been noted in some NUT carcinomas (D Bell unpublished observations).
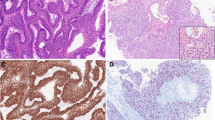
Salient morphological features of NUT carcinoma, with immunohistochemistry for NUT surrogate diagnosis, SOX2 and PRAME as promising anticancer targets, are illustrated in Fig. 1.
NUT carcinoma. (A, B) Salient morphological features-monotonous proliferation of round small-to-medium-sized cells; abrupt keratinization and foci of squamous differentiation with larger cells with eosinophilic cytoplasm and pearl formation (C). Immunoperoxidase staining with (D) anti-NUT, (E) anti-SOX2, (F) anti-PRAME
Sinonasal Lymphoepithelial Carcinoma (SLEC)
Similar to other anatomical sites, sinonasal lymphoepithelial carcinoma (SLEC) is composed of sheets of undifferentiated malignant epithelial cells intimately intermingled with chronic inflammatory infiltrate. Malignant cells are often EBV positive [33], as shown by in situ hybridization for EBV-encoded RNA (EBER); serology for EBV-encoded RNA is also available.
The presence of lymphocytic infiltration, EBV expression, and the lack of neuroendocrine markers helps to differentiate SLEC mainly from SNUC and HGNEC.
High expression of somatostatin receptor 2 (SSTR2) has been documented in nasopharyngeal lymphoepithelial carcinoma, thymic LEC and salivary LEC 34,35,36,37,38]. SSTR2, a G-protein-coupled cell surface receptor, inhibits cell proliferation and is mainly expressed in neuroendocrine tumors. Lechner et al., in their large cohort of nasopharyngeal carcinomas, proposed a prognostic role for SSTR2 expression, with higher expression associated with increased survival rates [35]. High expression of SSTR2 is helpful as a diagnostic biomarker by imaging and an increased uptake of specific radiocontrast in EBV + NPC [39]. Targeted therapeutic strategies with SSTR2 agonists have also been studied with agonists prolonging progression-free survival in patients with metastatic enteropancreatic neuroendocrine tumors grade 1 or 2 (Ki67 < 10%) [40].
In view of SSTR2 diagnostic, imaging and therapeutic implications extrapolated from nasopharyngeal carcinoma studies, along with SSTR2 sensitivity and specificity for LEC, SSTR2 testing in SLECs is encouraged. Figure 2 depicts a nasal septum EBER-positive undifferentiated carcinoma, with strong expression of PRAME and SSTR2.
Sinonasal Undifferentiated Carcinoma (SNUC) and IDH2-Mutant Sinonasal Carcinomas
The WHO redefined SNUC as a highly aggressive and clinicopathologically distinct carcinoma of uncertain histogenesis that typically presents with great local aggressivity and tendency to metastasize [41]. SNUC is reputed to be refractory to even the most radical therapy and to carry a poor prognosis, particularly when the tumor transgresses the cranial base [42, 43].
In general, SNUC presents high chemosensitivity to cisplatin-based regimens, and a partial or complete response to induction chemotherapy is considered a favorable prognostic factor. Definitive chemoradiation is therefore usually recommended, while surgery is used as a salvage treatment in cases of persistence or recurrence [44].
SNUC arises from the sinonasal epithelium and therefore is of ectodermal derivation. In light of the overlapping clinical, anatomical, microscopic, and ultrastructural findings in olfactory neuroblastoma (ONB) and neuroendocrine carcinoma (NEC), their origins may share both cells of sinonasal respiratory mucosa and cells of olfactory neuroepithelium [45]. It has also been proposed that SNUC would be best categorized as a large-cell neuroendocrine carcinoma (reviewed in [46]).
SNUC is regarded as a diagnosis of exclusion. The immunohistochemical panel stains positively for epithelial markers (AE1/AE3, CK7, CAM5.2, EMA), p16, CD117, and focal p63 and negatively for CK5/6, p40, CEA, EBER, CD34, desmin, S100 protein, and calretinin. Neuroendocrine markers (synaptophysin, chromogranin, INSM, CD56) may be present.
An SNUC subtype with mutations in the Krebs cycle enzyme IDH2 is well characterized. IDH2 p.R172S is the most common mutation (55%); other mutations in the same codon (R172M, R172T, and R172G) have been described, and IDH1 mutations have rarely been reported [41, 47]. The spectrum is expanding, with IDH2 mutations documented in poorly differentiated high-grade carcinomas occurring in the sinonasal/paranasal anatomical boundaries as well as a handful of high-grade olfactory neuroblastomas [48]. As hypermethylation and upregulation of the repressive H3K27 epigenetic mark are hallmarks of IDH2-mutated carcinomas, DNA methylation-based classification is conceivable [48]. Given the therapeutic implications of IDH inhibitors, paralleling acute myeloid leukemia, some authors advocate for the classification of IDH2-mutated sinonasal tumors as a separate entity.
To date, no morphological or phenotypical differences between IDH-mutant and IDH-WT carcinomas have been recognized. Antibodies that recognize IDH1/2 (pR132/172) are a surrogate for diagnosis confirmation (granular cytoplasmic staining pattern); however, molecular testing validation is recommended. An example of a maxillary SNUC IDH2 mutation is shown in Fig. 3; the genomic event was confirmed by NGS studies.
SWI/SNF Complex-Deficient Sinonasal Carcinomas
Sinonasal carcinomas characterized by rhabdoid/basaloid morphology and loss of expression of the SWI/SNF complex (SMARCB1, SMARCA4, SMARCA2), previously viewed as a subset of SNUCs, are recognized as a standalone entity in the 5th edition WHO Classification of Head and Neck Tumours [49]. Separation from the other types of sinonasal malignancies is justified, as the identification of SWI/SNF complex deficiency may provide a new target for novel treatment approaches and may ultimately lead to improved patient survival [50].
Available antibodies for SMARCB1/INI1 (BAF47) and SMARCA4 (BRG1) are routinely employed surrogates (FISH and NGS molecular studies offer verification of these genomic alterations). The tumor is positive for pancytokeratin and variably positive for CK5/6, p63/p40, and CK7; focal reactivity for synaptophysin and chromogranin is evident. P16 immunostaining is often positive but is not associated with the presence of HPV. HPV, EBV, and NUT are negative. Complete loss of SMARCB1 (INI1) is mandatory (SMARCA4 expression is retained); conversely, loss of SMARCA4 (with preservation of SMARCB-1/INI1) is diagnostic (Fig. 4a, b). Co-loss of SMARCA2 is occasionally observed [51].
SNI/SWI complex-deficient sinonasal carcinoma. (a) SMARCB1/INI1-deficient sinonasal carcinoma. (A). H&E. Undifferentiated carcinoma (with large nuclei and prominent nucleoli) (B) Diffuse immunoreactivity with anti-p16. (C) Complete loss of expression of SMARCB1/INI1 (immunoperoxidase study with anti INI1/BAF47, with internal positive control/endothelial cells). (D) Patchy and weak expression of synaptophysin. E) Diffuse loss of expression of PTEN (correlated with NGS findings). (b) SMARCA4-deficient sinonasal carcinoma. (A, B) H&E- High-grade rhabdoid cells and rhabdoid appearance. (C) Complete loss of expression of SMARCA4 visualized by immunostaining with anti-BRG1 (vascular internal control highlights nuclear signal and retention)
The spectrum of SWI/SNF-deficient sinonasal carcinomas currently includes the following: (1) SMARCB1-deficient sinonasal carcinoma, (2) SMARCB1-deficient sinonasal adenocarcinoma (with unequivocal glands or yolk–sac pattern), (3) SMARCA4 undifferentiated carcinoma, and (4) SMARCA4-deficient subset of teratocarcinosarcoma [51].
Sinonasal Nonkeratinizing Squamous Cell Carcinoma (SNKSCC) NOS
A morphologically distinct sinonasal carcinoma (prior terminology as transitional, cylindrical cell, Schneiderian, and Ringertz carcinoma) is composed of cytologically atypical neoplastic cells arranged in ribbons that lack maturation and significant keratinization. Two subtypes have been added to the 5th edition WHO Classification of Head and Neck Tumours [52]: (i) HPV-associated – NKSCC defined by the presence of transcriptionally active HPV high risk and (ii) the emerging entity of DEK::AFF2 NKSCC characterized by recurrent DEK::AFF2 fusions 53,54,55]. Morphological differences between NKSCC-NOS and these subtypes of carcinomas are not appreciated.
SNKSCCs are diffusely positive for keratins CK5/6 and 34ß12 (CK903) and for p63 and p40; negative for synaptophysin, chromogranin, and INSM1, although occasional discrete or focal positivity for neuroendocrine markers is accepted; negative for NUT and EBV; and show retained SMARC expression. An example of NKSCC-NOS is shown in Fig. 5. Methodologies for DEK::AFF2 fusion confirmation include RNA sequencing, DEK FISH, or surrogate AFF2 antibody [56].
Sinonasal nonkeratinizing SCC, NOS. (A, B). H&E Nonkeratinizing squamous cell carcinoma with ribboned arrangement (A) and discrete keratinization (B- 200 × magnification). (C) Diffuse positivity with anti-p40 antibody and (D) scattered anti-INSM1 reactivity. (E) PRAME is diffusely expressed within tumor cells. Viral studies (EBER, HPV-hr) are negative (not illustrated)
Sinonasal Teratocarcinosarcoma and Sinonasal High-Grade Poorly Differentiated Sinonasal Carcinomas NOS
Sinonasal Teratocarcinosarcoma (STCS)
TCS is a rare skull base and sinonasal tract malignant tumor composed of carcinomatous, sarcomatous, and immature neural elements [57]. The most frequent sites are the ethmoid and maxillary sinuses and the nasal cavity in elderly male patients [58].
Morphologically, the tumor is characteristically composed of a high-grade carcinomatous component admixed with sarcomatous and immature neural elements. This tumor causes a diagnostic dilemma if only a dominant component is present on small biopsy samples. The TCS phenotype mirrors its constituent components: cytokeratin immunoreactivity within the epithelial component, CK5/6, p40, p63 for squamous elements, conventional neuroendocrine markers (chromogranin, synaptophysin, INSM1) highlighting the neuroepithelial component (and occasionally focally positive in epithelial), reactivity for myogenic markers (desmin, MyoD1, myogenin), SATB2, and SOX9 in sarcoma elements. Markers of germ cell derivation AFP, PLAP, and hCG are usually negative; however, positivity for SALL4 can be observed, and SALL4 immunohistochemistry appears to be relatively sensitive and specific for the diagnosis of TCS [59, 60]. Recurrent SMARCA4 alterations resulting in loss of SMARCA4 (BRG1) have been documented in up to 70% of studied TCS cases [61, 62]. Aberrant nuclear ß-catenin localization has been reported in a subset of TCS [61].
Despite major technological advances instrumental in refining the classification of sinonasal carcinomas, high-grade poorly differentiated sinonasal carcinoma NOS constitutes a temporary default diagnosis for a subset of cases. Figure 6 offers an illustrative example of a high-grade carcinoma arising from the middle turbinate in a middle-aged man (case from author files). The histological appearance is dominated by the presence of surface epithelial dysplastic transformation with endophytic epithelial growth of complex architectural patterns (ribboned, glandular, sieve-like spaces, ciliated neoplastic epithelium, and Schiller-Duval-like elements). No sarcoma elements are identified upon thorough sampling and examination of surgical specimens. The lack of all traditional neuroendocrine and conventional germ cells (AFP, PLAP, glypican, hCG) adds to the diagnostic challenges. SALL4 and PRAME expressions inform the pluripotential embryonic stem/germ cell origin. Comprehensive molecular NGS studies have not resulted in a more definitive diagnosis.
High-grade sinonasal (“Schneiderian”) carcinoma NOS (arising from the middle turbinate). (A–D) H&Es. The histological appearance is dominated by the presence of surface epithelial dysplastic transformation with endophytic epithelial growth of complex architectural patterns (ribboned, glandular, sieve-like spaces, ciliated neoplastic epithelium, and Schiller-Duval-like elements (D) Diffuse immunoreactivity with anti-CK7 (E) and limited CK5/6 expression (F). No sarcoma elements are identified upon thorough sampling and examination of surgical specimens. The lack of all traditional neuroendocrine and conventional germ cells (AFP, PLAP, glypican, hCG) adds to the diagnostic challenges. SALL4 (G) and PRAME (H) expressions inform the pluripotential embryonic stem/germ cell origin. Comprehensive molecular NGS studies have not resulted in a more definitive diagnosis
Sinonasal Neuroendocrine Carcinoma (SNEC)
Sinonasal neuroendocrine carcinomas (divided into small- and large-cell carcinomas) are almost always high-grade tumors with morphological and immunohistochemical evidence of neuroendocrine differentiation, characterized by a dismal prognosis and a high tendency to produce systemic metastasis. The most common location is the nasal cavity (40%), followed by the ethmoid sinus and maxillary sinuses (~ 20%), sphenoid sinus (13%), and frontal sinus (2%).
The 5th edition WHO Classification of Head and Neck Tumours relocates sinonasal small-cell neuroendocrine carcinomas [63] (SmCNECs) and large-cell neuroendocrine carcinomas [64] (LCNECs) into a dedicated neuroendocrine tumor section in an effort to unify neuroendocrine tumor terminology across organ systems. Regardless, their diagnostic criteria have not changed, with a minimum of 10 mitoses per 2 mm2 and Ki67 > 20% being mandatory.
The immunohistochemical profile includes positivity for cytokeratins AE1/AE3, Cam5.2, CK8/18 (frequently with a perinuclear dot distribution), and neuroendocrine markers (synaptophysin, chromogranin, INSM1- variable expression). The Ki67 mitotic index is more than 20%, usually ~ 70–80%). SNECs may benefit from induction chemotherapy followed by concurrent chemoradiation; surgery can be performed in nonresponsive cases or as a salvage treatment.
There is an ongoing effort to apply and validate novel lung small-cell (SCLC) molecular subtyping and biomarker-driven therapy for SNEC. According to RNA expression with validation at the protein levels of the transcription factors ASCL1, NEUROD1, POU2F3, and YAP1, four SCLC subtypes have emerged: SCLC-A (ASCL1-driven), SCLC-N (NEUROD1-driven), SCLC-P (ASCL1/NEUROD1-double negative with POU2F3 expression), and SCLC-Y (YAP1-related and NOS) and SCLC-I (inflamed gene signature), which share the last subtype 65,66,67,68,69]. SCLC-Is exhibit the greatest response to the addition of immunotherapy to chemotherapy, while the other subtypes each have distinct vulnerabilities, including to inhibitors of PARP, Aurora kinases, or BCL-2 [68].
Achaete-scute homolog 1 (ASCL1) is a powerful player in modulating neuroendocrine differentiation in tumor cells. ASH1 expression levels are inversely associated with the degree of tumor differentiation (high-grade tumors show increased expression of this protein), which correlates well with studies indicating that the expression of ASCL1 appears to be restricted to immature cells 70,71,72,73]. ASCL1 expression has been documented previously in high-grade carcinomas of the sinonasal tract [32, 74]. Figure 7 depicts a nasal cavity SLCNEC with classical morphology and diffuse ASCL1 expression.
Sinonasal large-cell neuroendocrine carcinoma (LCNEC). (A–C) H&Es. Classical morphology- large cell size (> 3 lymphocytes), granular, stippled chromatin; architecture- organoid, peripheral palisading, trabeculae, pseudorosettes; large confluent central necrosis, mitoses (typically > 10 MF/2 mm2) (D). Diffuse immunoreactivity with anti-Cam5.2 (including dot pattern) and (E) diffuse ASCL1 expression
Neurogenic differentiation factor 1 (NEUROD1)-high SCLC is associated with higher overall neuroendocrine marker expression, equivalent to that of ASCL1-dominant tumors [65, 67, 68].
POU class 2 homeobox 3 (POU2F3) is a marker of chemosensory tuft cells (brush cells in the lung airways), and its expression is associated with low expression levels of neuroendocrine markers in lung SCLC. Yes-associated protein 1 (YAP1), a transcription regulator in the HIPPO growth signaling pathway, was found to be expressed in a subset of nonneuroendocrine SCLCs [65, 67, 68]. Koh et al. recently showed that molecular classification of SCLCs can be applied to extrapulmonary neuroendocrine carcinomas/poorly differentiated carcinomas (EP-NEC/PDCs) and that POU2F3-dominant or YAP1-dominant subtypes are distinct subtypes of EP-NEC/PDCs [75].
Neuroectodermal Neoplasms
Olfactory Neuroblastoma (ONB) and Olfactory Carcinoma
ONB represents the “sine-qua-non” for SRBCTs, arranged in submucosal lobules with a neurofibrillary background set in a richly vascular or hyalinized stroma. Immunohistochemical profiles including a spectrum of different cell lineages are crucial to the diagnosis. ONB is usually diffusely positive for chromogranin and synaptophysin, with S100 protein-positive sustentacular cells characteristically highlighting the periphery of tumor lobules. Sustentacular cells tend to be attenuated/disappear with histological grading progression. Negative staining includes muscle markers, leukocyte common antigen CD45 (LCA), CD99, and p40. Up to one-third of ONBs will exhibit focal staining for cytokeratin (most common are low molecular keratins Cam 5.2 and CK 8/18). ONB can express somatostatin receptors (SSTR2 in particular); somatostatin analogs can be used for diagnosis, especially in cases of metastatic disease [76, 77]. Recently, Zunitch et. Al, utilized an integrated human-mouse single cell atlas of the nasal mucosa, including the olfactory epithelium, to identify transcriptomic programs that link ONB to a specific population of stem,/progenitor cells – olfactory globose basal cells (GBCs) [78]. The authors further advocate that expression of a GBC transcription factor (NEUROD1) distinguishes both low-and high-grade ONB from SNUC. Furthermore, their study identified a reproducible subpopulation of highly proliferative ONB cells expressing the GBC stemness marker EZH2, suggesting that EZH2 inhibition may play a role in the targeted treatment of ONB [78].
ONB exhibits epithelial differentiation to varying degrees, which is a unique feature that sets it apart from peripheral neuroblastoma in other regions; melanocytic, myogenic, and neural differentiation has been occasionally reported [79]. These are manifested in the forms of glands, squamous morules, and rhabdomyoblastic or ganglioneuroblastic differentiation. Divergence may be encountered in pretreatment or posttreatment samples and can change after treatment; such divergence should be accepted only when a pathognomonic feature of ONB is identified (neurofibrillary stroma or sustentacular cells) or in a recurrence/posttreatment resection of an otherwise typical ONB [46].
Rooper et al. reviewed a total of 53 sinonasal tumors with neuroepithelial differentiation [79]. The majority of these arose in the superior nasal cavity and high stage (Kadish-Morita) at presentation. Morpho-phenotypical findings included (i) lobulated and solid growth; (ii) rosettes and/or neurofibrillary stroma; (iii) high-grade cytology; (iv) glands, frequently ciliated; and (v) extensive keratin and neuroendocrine immunoreactivity, variable sustentacular S100 component. The authors advocated for a unifying nomenclature as re-instauration of the olfactory carcinoma taxonomy, with olfactory carcinoma being defined by high-grade keratin-positive neuroectodermal cells with frequent intermixed glands and recurrent Wnt pathway, ARID1A, and RUNX1 alteration [80, 81]. Figure 8 illustrates olfactory carcinoma.
Olfactory carcinoma. Morpho-phenotypical findings included (i) lobulated and solid growth; (ii) rosettes and/or neurofibrillary stroma; (iii) high-grade cytology; (iv) glands, frequently ciliated (A–C H&Es); and (v) extensive keratin and neuroendocrine immunoreactivity, variable sustentacular S100 component. Immunoperoxidase staining with anti-synaptophysin (D), anti-S100, anti-Cam5.2 (F), anti-AE1/AE3 (G)
Malignant Mucosal Melanoma (MM)
Sinonasal MM is the most aggressive sinonasal tumor and is currently characterized by early recurrence and high dissemination rates regardless of the treatment adopted. Free-margin surgery is the mainstay of treatment since it is generally considered a radio-resistant cancer. Cells are histologically comparable to melanoma arising in other locations and may be plasmacytoid, epithelioid, spindle, or rhabdoid. Architectural patterns are varied and nonspecific, and pigmentation is variable. When lacking melanin pigment, immunohistochemistry becomes paramount: S100 protein, SOX10, and PRAME are usually strongly positive, while other melanocytic markers (HMB45, tyrosinase, melan A, and MITF) have variable expression. The well-known mutated genes involved in cutaneous melanoma have only a marginal role in MM, and the infrequent rate of BRAF V600E mutation observed in MM limits the efficacy of BRAF inhibitors. Recently, immunotherapy has shown promising results in selected cases, both in neoadjuvant and adjuvant settings, especially in terms of decreased systemic spread of disease [82].
Ectopic Pituitary Adenoma
Pituitary adenomas mainly occur in the sphenoid bone and sinuses either as a separate lesion or as an extension from a primary adenoma arising in the sella; embryonic residue along the Rathke pouch formation is the presumed derivation [84]. Approximately half of patients manifest hormonal abnormalities. Histologically, an ectopic pituitary adenoma is identical to a conventional pituitary adenoma with monotonous round cells.
This neoplasm should be differentiated from carcinoid tumors, olfactory neuroblastoma, and other small undifferentiated tumors at these locations, as the expression of Cam5.2 is a common pitfall. Immunohistochemical staining for hormonal receptors, especially for ACTH and prolactin, and pituitary transcription factors Pit1 and T-pit is very helpful (Fig. 9).
Ectopic pituitary adenoma. (A–C) H&Es. Histologically, an ectopic pituitary adenoma is identical to a conventional pituitary adenoma with monotonous round cells. This neoplasm should be differentiated from carcinoid tumors, olfactory neuroblastoma, and other small undifferentiated tumors at these locations, as the expression of Cam5.2 is a common pitfall (D); diffuse positivity for synaptophysin (E) with folliculostellate (FSC) cells highlighted by S100 (F); proliferation rate Ki67 is low, 2% (G). Immunohistochemical staining for hormonal receptors, especially for ACTH (H) and pituitary transcription factor Pit1 (I), is helpful
Ewing Family Tumors (EFTs)
Ewing family tumors (previously known as Ewing sarcoma (EWS) and primitive neuroectodermal tumor (PNET)) are interrelated primitive round cell malignancies of neuroectodermal derivation. They represent a spectrum of morphologic entities that share common molecular genetic features, e.g., fusions involving members of the FET and ETS gene families. They are uncommon childhood and young adult tumors affecting the skull base and sinonasal tract regions in approximately 5% of patients. The maxillary sinus and the nasal fossa are commonly affected sites. Histologically, the tumor presents in sheets and nests of densely uniform small-cell proliferation, with occasional rosette formation. CD99 (MIC2) is diffusely positive (membranous pattern); nuclear expression of Fli-1 and NKX2.2 also supports the diagnosis; chromogranin, synaptophysin, or low-molecular-weight cytokeratin is expressed in a subset of EFTs. The adamantinoma-like variant is strongly positive for high-molecular-weight cytokeratin, p40 and p63. NGS (with EWSR1 or FUS rearrangement) and FISH for EWSR1 are confirmatory for diagnosis (supplemental Fig. 1).
Other SRCTs
Rhabdomyosarcoma (RMS)
This is a relatively uncommon mesenchymal malignancy of the skull base region. Rhabdomyosarcoma is the most common sarcoma of the head and neck and is the most frequent childhood sarcoma. The sinonasal tract and the nasopharynx are the most affected sites. The embryonal type (ERMS) is the most common type in children, while the alveolar type (ARMS) predominates in an older age group.
Immunohistochemical markers, including desmin, myo-D, and myogenin, are necessary for the diagnosis, especially of the embryonal form. Most alveolar subtype RMSs harbor a PAX3 or PAX7::FOXO1 fusion. A PAX3 variant translocation that partners with NCOA family members in place of FOXO1 has been described in RMS [83].
Hematolymphoid Malignancies
Plasmablastic Lymphoma (PBL)
Plasmablastic lymphoma is a high-grade B-cell lymphoma with a plasmacytoid appearance and plasma cell phenotype and is frequently associated with MYC overexpression. The sinonasal tract may be affected, as this lymphoma occurs predominantly at extranodal sites. The tumor cells express plasma cell markers (CD38, MUM1, CD138) and light chain restriction (either kappa or lambda); B-cell markers (CD20, PAX5) are negative; and EBER is positive (at least in 50% of cases) (supplemental Fig. 2).
Extramedullary Plasmacytoma
A similar morphology and phenotype make extramedullary plasmacytoma challenging to distinguish from PBL. Extramedullary plasmacytoma is defined by the absence of bone marrow involvement and end organ damage, which are found in PBL. Diagnostic confirmation can be achieved by immunohistochemistry or in situ hybridization for immunoglobulin mRNA with identification of light chain restriction. The majority of plasmacytomas are negative for EBER and have no MYC rearrangements. Where distinction between PBL and plasmacytoma is not possible, a descriptive diagnosis of plasmablastic neoplasm is suggested (supplemental Fig. 2).
Conclusions
The diagnostic challenges of sinonasal SRBCTs have been addressed to date by introducing newly described entities, reclassifying conventional lesions, and redesigning treatment modalities for these rare tumors after conducting multi-institutional cohort retrospective analysis. The accuracy of diagnosis is sine-qua-non for the therapeutic approach and prognosis of patients with sinonasal cancer.
The initial goal is to establish the lineage and triage the tissue for ancillary and molecular studies. Ideally, differentiation is reached based on multiple factors, including more than one stain. If the result is equivocal, rather than interpreting as “weak positive/essentially negative, additional confirmatory studies should be performed. Awareness of “aberrant expression” is important to avoid confusion. Many diagnostic errors occur because too few markers are assessed. Figure 10 illustrates a routine ancillary work-up algorithm for sinonasal undifferentiated/SRBCT.
The request for specific molecular testing is based on differential diagnostic considerations and implies a continuous screening of literature and updates. The lack of access for pathologists worldwide to the latest technologies and the limited nature of these SRBCTs (with an inability to perform extensive exploratory testing) are several drawbacks. Table 1 summarizes a wish list of diagnostic “hacks” and theragnostic ancillary studies. Laforga and Abdullah recently formalized a diagnostic algorithm for these tumors by integrating morphological clues, immunohistochemical markers and morphological investigations [85]. The algorithm is presented through a user-friendly web interface and a mobile phone application, with the intent to help pathologists establish a correct diagnosis of a challenging SRBCT and researchers perform retrospective analysis of archival cases. Prior to this, several algorithms based on another single institutional experience have been proposed to meet the need for integrated diagnosis for skull base malignancies [86, 87].
Sinonasal SRCTs are rare and heterogeneous tumors, with an imperative need for novel diagnostic, prognostic, and therapeutic biomarkers. Validation of the current findings and building a comprehensive model of carcinogenesis for each sinonasal tumor require multi-institutional efforts. With more novel targeted therapies being developed, options for personalized treatment of sinonasal cancers are growing, with the goal of improved survival for this challenging group of tumors.
Data Availability
Not applicable.
Code Availability
Not applicable.
Change history
18 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12105-024-01636-3
References
Hermsen MA, Bossi P, Franchi A, Lechner M (2023) Sinonasal cancer: improving classification, stratification and therapeutic options. Cancers (Basel). 15(6):1675
Hermsen MA, Riobello C, Garcia-Marin R et al (2020) Translational genomics of sinonasal cancers. Semin Cancer Biol 61:101–109
Simons SA, Bridge JA, Leon ME (2016) Sinonasal small round blue cell tumors: an approach to diagnosis. Semin Diagn Pathol 33(2):91–103
French C (2014) NUT midline carcinoma. Nat Rev Cancer 14(3):149–150
French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA (2003) BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res 63(2):304–307
French CA, Ramirez CL, Kolmakova J et al (2008) BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 27(15):2237–2242
French CA, Rahman S, Walsh EM et al (2014) NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov 4(8):928–941
Shiota H, Elya JE, Alekseyenko AA et al (2018) “Z4” Complex member fusions in NUT carcinoma: implications for a novel oncogenic mechanism. Mol Cancer Res 16(12):1826–1833
Moreno V, Saluja K, Pina-Oviedo S (2022) NUT carcinoma: clinicopathologic features, molecular genetics and epigenetics. Front Oncol 12:860830
French CA, Minato H, Stelow ES et al (2022) NUT carcinoma. In: WHO Classification of Tumours Editorial Board (ed) WHO classification of head and neck tumours. International Agency for Research on Cancer, Lyon
Haack H, Johnson LA, Fry CJ et al (2009) Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol 33(7):984–991
Maur M, Toss A, Dominici M et al (2015) Impressive response to dose-dense chemotherapy in a patient with NUT midline carcinoma. Am J Case Rep 16:424–429
Patel SA, Singer B, Shen C, Zanation AM, Yarbrough WG, Weiss J (2021) A case of metastatic NUT carcinoma with prolonged response on gemcitabine and nab-paclitaxel. Clin Case Rep 9(8):e04616
Schwartz BE, Hofer MD, Lemieux ME et al (2011) Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res 71(7):2686–2696
Grayson AR, Walsh EM, Cameron MJ et al (2014) MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene 33(13):1736–1742
Boyer LA, Lee TI, Cole MF et al (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122(6):947–956
Wang R, Liu W, Helfer CM et al (2014) Activation of SOX2 expression by BRD4-NUT oncogenic fusion drives neoplastic transformation in NUT midline carcinoma. Cancer Res 74(12):3332–3343
Gangemi RM, Griffero F, Marubbi D et al (2009) SOX2 silencing in glioblastoma tumor-initiating cells causes stop of proliferation and loss of tumorigenicity. Stem Cells 27(1):40–48
Leis O, Eguiara A, Lopez-Arribillaga E et al (2012) Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene 31(11):1354–1365
Sanada Y, Yoshida K, Ohara M, Oeda M, Konishi K, Tsutani Y (2006) Histopathologic evaluation of stepwise progression of pancreatic carcinoma with immunohistochemical analysis of gastric epithelial transcription factor SOX2: comparison of expression patterns between invasive components and cancerous or nonneoplastic intraductal components. Pancreas 32(2):164–170
Freier K, Knoepfle K, Flechtenmacher C et al (2010) Recurrent copy number gain of transcription factor SOX2 and corresponding high protein expression in oral squamous cell carcinoma. Genes Chromosomes Cancer 49(1):9–16
Lopez L, Fernandez-Vanes L, Cabal VN et al (2023) Sox2 and betaIII-tubulin as biomarkers of drug resistance in poorly differentiated sinonasal carcinomas. J Pers Med. 13(10):1504
Gonzalez-Marquez R, Llorente JL, Rodrigo JP et al (2014) SOX2 expression in hypopharyngeal, laryngeal, and sinonasal squamous cell carcinoma. Hum Pathol 45(4):851–857
Schrock A, Goke F, Wagner P et al (2013) Sex determining region Y-box 2 (SOX2) amplification is an independent indicator of disease recurrence in sinonasal cancer. PLoS ONE 8(3):e59201
Dorna D, Paluszczak J (2023) Targeting cancer stem cells as a strategy for reducing chemotherapy resistance in head and neck cancers. J Cancer Res Clin Oncol 149(14):13417–13435
Balachandran S, Narendran A (2023) The developmental origins of cancer: a review of the genes expressed in embryonic cells with implications for tumorigenesis. Genes (Basel). 14(3):604
Finn OJ (2008) Cancer immunology. N Engl J Med 358(25):2704–2715
Finn OJ (2017) Human tumor antigens yesterday, today, and tomorrow. Cancer Immunol Res 5(5):347–354
Finn OJ (2018) A believer’s overview of cancer immunosurveillance and immunotherapy. J Immunol 200(2):385–391
Xu Y, Zou R, Wang J, Wang ZW, Zhu X (2020) The role of the cancer testis antigen PRAME in tumorigenesis and immunotherapy in human cancer. Cell Prolif 53(3):e12770
Salmaninejad A, Zamani MR, Pourvahedi M, Golchehre Z, Hosseini Bereshneh A, Rezaei N (2016) Cancer/testis antigens: expression, regulation, tumor invasion, and use in immunotherapy of cancers. Immunol Invest 45(7):619–640
Bell D, Bell A, Ferrarotto R et al (2020) High-grade sinonasal carcinomas and surveillance of differential expression in immune related transcriptome. Ann Diagn Pathol 49:151622
Hsieh SV, Stevens TM (2022) Sinonasal lymphoepithelial carcinoma. In: WHO Classification of Tumours Editorial Board (ed) WHO classification of head and neck tumours. International Agency for Research on Cancer, Lyon
Adili A, OConnor T, Wales P et al (2023) (2023) Challenging tumor heterogeneity with HER2, p16 and somatostatin receptor 2 expression in a case of ebv-associated lymphoepithelial carcinoma of the salivary gland. Head Neck Pathol. 17(4):1052
Lechner M, Schartinger VH, Steele CD et al (2021) Somatostatin receptor 2 expression in nasopharyngeal cancer is induced by Epstein Barr virus infection: impact on prognosis, imaging and therapy. Nat Commun 12(1):117
Loh KS, Waser B, Tan LK, Ruan RS, Stauffer E, Reubi JC (2002) Somatostatin receptors in nasopharyngeal carcinoma. Virchows Arch 441(5):444–448
Roden AC, Rakshit S, Johnson GB, Jenkins SM, Mansfield AS (2022) Correlation of somatostatin receptor 2 expression, 68Ga-DOTATATE PET scan and octreotide treatment in thymic epithelial tumors. Front Oncol 12:823667
Viswanathan K, Sadow PM (2021) Somatostatin receptor 2 is highly sensitive and specific for Epstein-Barr virus-associated nasopharyngeal carcinoma. Hum Pathol 117:88–100
Schartinger VH, Dudas J, Url C et al (2015) (68)Ga-DOTA (0)-Tyr (3)-octreotide positron emission tomography in nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging 42(1):20–24
Caplin ME, Pavel M, Cwikla JB et al (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371(3):224–233
Jo V, Agaimy A, Franchi A et al (2022) Sinonasal undifferentiated carcinoma. In: WHO Classification of Tumours Editorial Board (ed) WHO classification of head and neck tumours. International Agency for Research on Cancer, Lyon
Abdelmeguid AS, Bell D, Hanna EY (2019) Sinonasal undifferentiated carcinoma. Curr Oncol Rep 21(3):26
Abdelmeguid AS, Bell D, Hanna EY (2020) Neuroendocrine carcinoma and sinonasal undifferentiated carcinoma. Adv Otorhinolaryngol 84:168–184
Amit M, Abdelmeguid AS, Watcherporn T et al (2019) Induction chemotherapy response as a guide for treatment optimization in sinonasal undifferentiated carcinoma. J Clin Oncol 37(6):504–512
Mills SE (2002) Neuroectodermal neoplasms of the head and neck with emphasis on neuroendocrine carcinomas. Mod Pathol 15(3):264–278
Bell D (2018) Sinonasal neuroendocrine Neoplasms: current challenges and advances in diagnosis and treatment, with a focus on olfactory neuroblastoma. Head Neck Pathol 12(1):22–30
Jo VY, Chau NG, Hornick JL, Krane JF, Sholl LM (2017) Recurrent IDH2 R172X mutations in sinonasal undifferentiated carcinoma. Mod Pathol 30(5):650–659
Dogan S, Vasudevaraja V, Xu B et al (2019) DNA methylation-based classification of sinonasal undifferentiated carcinoma. Mod Pathol 32(10):1447–1459
Agaimy A, Bal MM, Jain D et al (2022) SWI/SNF-complex deficient sinonasal carcinoma. In: WHO Classification of Tumours Editorial Board (ed) WHO classification of head and neck tumours. International Agency for Research on Cancer, Lyon
Agaimy A, Franchi A, Lund VJ et al (2020) Sinonasal undifferentiated carcinoma (SNUC): from an entity to morphologic pattern and back again-a historical perspective. Adv Anat Pathol 27(2):51–60
Agaimy A (2023) SWI/SNF-deficient sinonasal carcinomas. Adv Anat Pathol 30(2):95–103
Rooper LM, Ihler S, Kiss K et al (2022) Non-keratinizing squamous cell carcinoma. In: WHO Classification of Tumours Editorial Board (ed) WHO classification of head and neck tumours. International Agency for Research on Cancer, Lyon
Kuo YJ, Lewis JS Jr, Zhai C et al (2021) DEK-AFF2 fusion-associated papillary squamous cell carcinoma of the sinonasal tract: clinicopathologic characterization of seven cases with deceptively bland morphology. Mod Pathol 34(10):1820–1830
Rooper LM, Agaimy A, Dickson BC et al (2021) DEK-AFF2 carcinoma of the sinonasal region and skull base: detailed clinicopathologic characterization of a distinctive entity. Am J Surg Pathol 45(12):1682–1693
Ruangritchankul K, Sandison A (2023) DEK::AFF2 fusion carcinomas of head and neck. Adv Anat Pathol 30(2):86–94
Kuo YJ, Lewis JS Jr, Truong T et al (2022) Nuclear expression of AFF2 C-terminus is a sensitive and specific ancillary marker for DEK::AFF2 carcinoma of the sinonasal tract. Mod Pathol 35(11):1587–1595
Rooper LM, Agaimy A, Bal MM et al (2022) Teratocarcinosarcoma. In: WHO Classification of Tumours Editorial Board (ed) WHO classification of head and neck tumours. International Agency for Research on Cancer, Lyon
Smith SL, Hessel AC, Luna MA, Malpica A, Rosenthal DI, El-Naggar AK (2008) Sinonasal teratocarcinosarcoma of the head and neck: a report of 10 patients treated at a single institution and comparison with reported series. Arch Otolaryngol Head Neck Surg 134(6):592–595
Compton ML, Lewis JS Jr, Faquin WC, Cipriani NA, Shi Q, Ely KA (2022) SALL-4 and beta-catenin expression in sinonasal teratocarcinosarcoma. Head Neck Pathol 16(1):229–235
Ito K, Yano M, Ogasawara A, Miwa M, Kozawa E, Yasuda M (2019) A unique uterine cervical “teratocarcinosarcoma”: a case report. Diagn Pathol 14(1):122
Rooper LM, Agaimy A, Gagan J et al (2023) Comprehensive molecular profiling of sinonasal teratocarcinosarcoma highlights recurrent SMARCA4 inactivation and CTNNB1 mutations. Am J Surg Pathol 47(2):224–233
Rooper LM, Uddin N, Gagan J et al (2020) Recurrent loss of SMARCA4 in sinonasal teratocarcinosarcoma. Am J Surg Pathol 44(10):1331–1339
Rooper LM, Classe M, Nose V et al (2022) Small cell neuroendocrine carcinoma. In: WHO Classification of Tumours Editorial Board (ed) WHO Classification of Head and Neck Tumours. International Agency for Research on Cancer, Lyon
Rooper LM, Classe M, Nose V et al (2022) Large cell neuroendocrine carcinoma. In: WHO Classification of Tumours Editorial Board (ed) WHO Classification of Head and Neck Tumours. International Agency for Research on Cancer, Lyon
Baine MK, Hsieh MS, Lai WV et al (2020) SCLC subtypes defined by ASCL1, NEUROD1, POU2F3, and YAP1: a comprehensive immunohistochemical and histopathologic characterization. J Thorac Oncol 15(12):1823–1835
Borromeo MD, Savage TK, Kollipara RK et al (2016) ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep 16(5):1259–1272
Denize T, Meador CB, Rider AB et al (2023) Concordance of ASCL1, NEUROD1 and POU2F3 transcription factor-based subtype assignment in paired tumour samples from small cell lung carcinoma. Histopathology
Gay CM, Stewart CA, Park EM et al (2021) Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 39(3):346-360 e347
Sung S, Heymann JJ, Politis MG, Baine MK, Rekhtman N, Saqi A (2022) Small biopsy and cytology of pulmonary neuroendocrine neoplasms: brief overview of classification, immunohistochemistry, molecular profiles, and world health organization updates. Adv Anat Pathol 29(6):329–336
Augustyn A, Borromeo M, Wang T et al (2014) ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A 111(41):14788–14793
Fujiwara T, Hiramatsu M, Isagawa T et al (2012) ASCL1-coexpression profiling but not single gene expression profiling defines lung adenocarcinomas of neuroendocrine nature with poor prognosis. Lung Cancer 75(1):119–125
Jiang SX, Kameya T, Asamura H et al (2004) hASH1 expression is closely correlated with endocrine phenotype and differentiation extent in pulmonary neuroendocrine tumors. Mod Pathol 17(2):222–229
La Rosa S, Marando A, Gatti G et al (2013) Achaete-scute homolog 1 as a marker of poorly differentiated neuroendocrine carcinomas of different sites: a validation study using immunohistochemistry and quantitative real-time polymerase chain reaction on 335 cases. Hum Pathol 44(7):1391–1399
Taggart MW, Hanna EY, Gidley P, Weber RS, Bell D (2015) Achaete-scute homolog 1 expression closely correlates with endocrine phenotype and degree of differentiation in sinonasal neuroendocrine tumors. Ann Diagn Pathol 19(3):154–156
Koh J, Kim H, Moon KC et al (2023) Molecular classification of extrapulmonary neuroendocrine carcinomas with emphasis on POU2F3-positive tuft cell carcinoma. Am J Surg Pathol 47(2):183–193
Cracolici V, Wang EW, Gardner PA et al (2021) SSTR2 expression in olfactory neuroblastoma: clinical and therapeutic implications. Head Neck Pathol 15(4):1185–1191
Lechner M, Takahashi Y, Turri-Zanoni M et al (2022) Clinical outcomes, Kadish-INSICA staging and therapeutic targeting of somatostatin receptor 2 in olfactory neuroblastoma. Eur J Cancer 162:221–236
Zunitch MJ, Fisch AS, Lin B et al (2023) Molecular evidence for olfactory neuroblastoma aa a tumor of malignant globose basal cells. Modern Pathol 36(5):100122
Bell D, Classe M, Perez-Ordonez B et al (2022) Olfactory neuroblastoma. In: WHO Classification of Tumours Editorial Board (ed) WHO Classification of Head and Neck Tumours. Agency for Research on Cancer, Lyon
Rooper LM, Bishop JA, Faquin WC et al (2022) Sinonasal tumors with neuroepithelial differentiation (olfactory carcinoma): delineation of their pathologic and clinical features with insights into their relationship to olfactory neuroblastoma and sinonasal carcinoma. Am J Surg Pathol 46(8):1025–1035
Rooper LM (2023) Proceedings of the 2023 North American Society of Head and Neck Pathology Companion Meeting, New Orleans, LA, March 12, 2023: navigating new developments in high grade sinonasal neuroendocrine and neuroectodermal neoplasms. Head Neck Pathol 17(2):299–312
Lechner M, Takahashi Y, Turri-Zanoni M et al (2023) International multicenter study of clinical outcomes of sinonasal melanoma shows survival benefit for patients treated with immune checkpoint inhibitors and potential improvements to the current TNM staging system. J Neurol Surg B Skull Base 84(4):307–319
Thompson LD, Seethala RR, Muller S (2012) Ectopic sphenoid sinus pituitary adenoma (ESSPA) with normal anterior pituitary gland: a clinicopathologic and immunophenotypic study of 32 cases with a comprehensive review of the english literature. Head Neck Pathol 6(1):75–100
Sumegi J, Streblow R, Frayer RW et al (2010) Recurrent t(2;2) and t(2;8) translocations in rhabdomyosarcoma without the canonical PAX-FOXO1 fuse PAX3 to members of the nuclear receptor transcriptional coactivator family. Genes Chromosomes Cancer 49(3):224–236
Laforga JB, Abdullah B (2023) Diagnostic algorithm for challenging blue cell sinonasal carcinoma. Pathol Res Pract 248:154683
Hall MB, Rosen MR, Gill K, Nyquist GG (2016) Sinonasal malignancy: what to do with an unexpected pathology result? Am J Otolaryngol 37(5):473–476
Naunheim MR, Goyal N, Dedmon MM et al (2016) An algorithm for surgical approach to the anterior skull base. J Neurol Surg B Skull Base 77(4):364–370
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This review has no specific funding.
Author information
Authors and Affiliations
Contributions
DB performed literature review and prepared the manuscript. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The author certifies that she has no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethics Approval
Not applicable.
Consent to Participate for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The original version of this article unfortunately contained mistakes in the captions of figures 1, 2 and 3. The captions for the part figures E, F in figure 1, part figures D, E in figure 2 and part figure C in figure 3 were missing. They read wrongly as:
Fig. 1 NUT carcinoma. (A, B) Salient morphological features-monotonous proliferation of round small-to-medium-sized cells; abrupt keratinization and foci of squamous differentiation with larger cells with eosinophilic cytoplasm and pearl formation; (C, D) Immunoperoxidase staining with anti-SOX2 (C) and anti-PRAME (D), as promising anticancer targets
Fig. 2 Sinonasal lymphoepithelial carcinoma. (A) H&E- conventional morphology, with syncytial undifferentiated malignant cells set in a rich lymphoid background (Regaud pattern). (B) Presence of Epstein- Barr virus confirmed by EBER in situ hybridization. Immunostainings with (C) PRAME and (D) SSTR2
Fig. 3 Sinonasal IDH2- mutated carcinoma. (A) H&E- Submucosal lobules of undifferentiated malignant cells, with large nuclei and prominent nucleoli (B) H&E. Diffuse immunoreactivity with anti-mutant IDH1/2 pR132/172, confirmed by molecular NGS
They should have read as follows:
Fig. 1 NUT carcinoma. (A, B) Salient morphological features-monotonous proliferation of round small-to-medium-sized cells; abrupt keratinization and foci of squamous differentiation with larger cells with eosinophilic cytoplasm and pearl formation (C). Immunoperoxidase staining with (D) anti-NUT, (E) anti-SOX2, (F) anti-PRAME
Fig. 2 Sinonasal lymphoepithelial carcinoma. (A, B) H&E, (C) Presence of Epstein-Barr virus confirmed by EBER in situ hybridization. Immunostaining with (D) PRAME and (E) SSTR2
Fig. 3 Sinonasal IDH2-mutated carcinoma. (A) H&E- Submucosal lobules of undifferentiated malignant cells, with large nuclei and prominent nucleoli (B) H&E. Diffuse immunoreactivity with anti-mutant IDH1/2 pR132/172 (C)
The original article has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bell, D. Top IHC/ISH Hacks for and Molecular Surrogates of Poorly Differentiated Sinonasal Small Round Cell Tumors. Head and Neck Pathol 18, 2 (2024). https://doi.org/10.1007/s12105-023-01608-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12105-023-01608-z