Abstract
Admission of infants and children with cardiac disease to the neonatal (NICU) and pediatric ICU (PICU) is ever increasing in India (30–50 % of all admissions). The commonest indication for admission to the NICU or PICU is acute deterioration of cardiac disease. This includes: acute heart failure, hypercyanotic spells, arrhythmias, pericardial tamponade and sick cardiac neonates who need urgent intervention. Other increasingly frequent indications for ICU admission include heart failure with concomitant chest infection and impending respiratory failure and, severe cyanotic heart disease with various stroke syndromes. It is thus essential that a pediatrician be comfortable with the ICU management of such children and that low cost ICU modalities be utilized in order to reach out to as many children as feasible. It is heartening that there is renewed interest in inexpensive therapies like noninvasive ventilation and therapeutic hypothermia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The last two decades have witnessed tremendous strides in the overall care of infants and children with cardiac disease in India. This has been partly due to refinements in the intensive care management of these children. With increasing awareness among pediatricians and parents of what is feasible for children with cardiac disease today, there is an ever increasing number of admissions of infants and children with cardiac disease to the neonatal and pediatric intensive care units (ICU) in our country. The burden of congenital heart disease in India is huge – it is estimated that about 180–200,000 infants with congenital heart disease are born each year [1]. In addition, rheumatic heart disease and acute myocarditis add to the number of infants and children presenting with acute heart failure (HF). Both congenital heart disease and acquired heart disease (rheumatic, myocarditis) may present with acute deterioration warranting admission into the ICU. The proportion of admissions of infants and children with heart disease to the neonatal intensive care unit (NICU) and pediatric intensive care unit (PICU) vary between 30 and 50 % of all admissions. Thus, it is of paramount importance that the average pediatrician be comfortable with the ICU management and stabilization of these infants and children.
Thus, the purpose of this article is to demystify ICU management of infants and children with cardiac disease. The article will discuss various indications for admission to the ICU, goals of ICU care and current aspects of ICU management of cardiac infants and children. The article will also touch on some aspects of innovative care that can be delivered on “a shoe string” i.e., using “cost saving” modalities so that cardiac ICU can be accessible to many more children in our country.
Indications for Admission to the Intensive Care Unit
The commonest indication for admission to the NICU or PICU is acute deterioration of cardiac disease [2–4]. Usual indications for admission in the infant or older child constitute: acute pulmonary edema or severe acute heart failure, hypercyanotic spells or “tet” spells, tachyarrhythmia or bradyarrhythmias and pericardial tamponade needing urgent drainage due to acute pericardial collections. Acute heart failure (HF) is most often due to i) pulmonary over circulation secondary to large left to right shunts ii) acute valvar regurgitation, typically mitral or aortic or both or due to iii) acute decline in ventricular function as in myocarditis or cardiomyopathy or other forms of structural heart disease (typically left sided lesions – coarctation of aorta, critical aortic stenosis, ALCAPA – anomalous origin of left coronary artery from pulmonary artery) [5, 6].
Other increasingly frequent indications for ICU admission include: heart failure with concomitant chest infection and impending respiratory failure, severe cyanotic heart disease with cerebrovascular accident or brain abscess needing additional neuroprotective care [2].
Neonates requiring admission to the NICU are those with suspected “urgent” heart disease i.e., those neonates who need “early or urgent intervention”. These neonates typically present in a dramatic fashion with profound cyanosis, circulatory collapse or shock or cyanosis with heart failure or collapse [7].
Goals of ICU Care
It is very important to have clear time sensitive goals in ICU management of these fragile infants and children to minimize mortality and neuromorbidity [8, 9]. The main goal of ICU management is to rapidly and swiftly optimize perfusion and tissue oxygen delivery as much as is feasible. This may result in dramatic resolution of symptoms resulting in ICU discharge e.g., in some cases of acute myocarditis as the myocardial function improves. In other cases of structural heart disease ICU care mainly involves medical stabilization prior to emergency or elective cardiac surgery or catheter intervention.
Thus, the targets in the ICU involve swift restoration, if possible of important clinical and biochemical parameters of acute deterioration — 1) improvement in perfusion, assessed by capillary refill, tachycardia, tachypnea, chest signs (rales, crepitations), cyanosis, normalization of blood pressure 2) improvement in metabolic acidosis, lactate levels, and SvO2 (mixed venous saturations) when measured. Lactate levels of ≥ 8 mmol/L for longer than 2 h are associated with a grim prognosis and invariably result in death [9, 10]. The importance of clinical parameters as tools of hemodynamic assessment cannot be underestimated, the most important parameters being tachycardia and peripheral perfusion as measured by capillary refill. Prolonged tachycardia and persistently delayed capillary refill have been shown to be associated with increased mortality rates and neuromorbidity [9]. Similarly goal directed therapy targeting SvO2 of 70 % has been shown to be associated with survival benefit [11]. There is, thus evidence that those ICU units that set time sensitive goals and assiduously monitor therapy have better outcomes [9, 11].
ICU Management of Common Clinical Scenarios
Acute Heart Failure in the PICU
One of the commonest indications for admission to the PICU is acute heart failure (HF). Management principles involve traditional simple physiologic manoeuvres to enhance cardiac output and measures to reduce metabolic demand. Manoeuvres to increase cardiac output include 1) gentle preload optimization 2) inotropes and inodilators (dobutamine, milrinone) to enhance myocardial contractility and optimize afterload 3) normalization of metabolic parameters e.g., glucose, calcium and magnesium [3, 12, 13]. There is increasing data that the infant myocardium is sensitive to extracellular calcium for both systolic and diastolic function and it is important to restore normocalcemia as soon as possible [14, 15]. This is best done by calcium infusions rather than boluses of calcium [15].
One of the challenges is to improve cardiac output in the grossly fluid overloaded wet and swollen infant who is operating outside the Frank Starling curve (Fig. 1). These infants are resistant to standard doses of diuretics with little or no response to bolus diuretic therapy. Many such infants and children respond to a bolus frusemide followed by diuretic infusions (frusemide infusions) [16, 17]. Doses of frusemide infusions range from 0.2 mg/kg/h to 1 mg/kg/h. Those fluid loaded infants and children with acute kidney injury may also need dialysis with a peritoneal dialysis catheter.
An effective, simple and inexpensive method to improve the cardiac output, decongest the “fluid loaded” patients and reduce the metabolic demand is “nonpharmacologic method” based on well described cardiopulmonary interactions [18, 19]. This method involves institution of CPAP i.e., continuous positive airway pressure by noninvasive ventilation or PEEP or positive end expiratory pressure during formal controlled ventilation [19]. An increase in intrathoracic pressure has been shown to enhance cardiac output in children with left ventricular dysfunction. So, today, there is increasing evidence that timely use of CPAP and PEEP greatly improve cardiac output in patients with increased filling pressures i.e., those with increased left ventricular diastolic pressures i.e., increased LVEDP and systolic heart failure [19, 20]. Thus, infants and children with acute HF should be supported with non invasive ventilation using CPAP of 8–10 mm if there are clinical signs of increased work of breathing. CPAP may be delivered by nasal prongs, nasal endotracheal tube sited in the nasopharynx or a mask interface or by a bilevel positive airway pressure (BiPAP) in older children. Non invasive ventilation has the advantage of avoiding intubation and intubation related complications like ventilator associated pneumonia and ventilator dependence. Non invasive ventilation also has the advantage that it may be delivered outside of the PICU i.e., in the ward, freeing much needed PICU beds.
If there is little clinical improvement in the work of breathing or perfusion over 15–20 min then it is of great importance to intubate and support with assisted ventilation as early as possible. Dramatic resolution of florid pulmonary edema with improvement in hemodynamics and perfusion often occur with PEEP levels of 8–10 mm. Current ventilatory strategy in both LV dysfunction and left to right shunts include reliance of PEEP for alveolar recruitment and tissue oxygenation, rather than increased tidal volumes or pressures. The preference is to use pressure regulated volume controlled ventilation (PRVC) with PEEP (8-10 mm)– use whatever tidal volume that induces chest expansion, and then cut down tidal volumes as the infant or child improves. Large left to right shunts often have very high ventilatory needs due to pulmonary over circulation. So, important considerations during assisted ventilation in heart failure are avoidance of over ventilation, hypocarbia and lung injury just as in normal children. The ventilatory goal should be “gentle ventilation” accepting permissive hypercarbia-pCO2 ~ 45–50 mm [20, 21] using a ventilatory strategy that maintains lung volumes near functional residual capacity. High inhaled oxygen concentrations (FiO2) should be avoided in large left to right shunts and in situations of pulmonary over circulation [e.g., dextro-Transposition of the great arteries (dTGA) with large ventricular septal defects, single ventricle with increased pulmonary blood flow etc.] to avoid sudden oxygen related reduction in pulmonary vascular resistance (PVR) and worsening of pulmonary edema and heart failure [20]. [A reduction in pulmonary vascular resistance is associated with an increase in left to right shunting (Qp/Qs) or increased pulmonary over circulation].
In summary, factors that increase pulmonary blood flow – e.g., over ventilation, hypocarbia, alkalosis and excessive oxygen delivery – all of which reduce pulmonary vascular resistance should be avoided during assisted ventilation in infants and children with large left to right shunts. In fact, modest hypercarbia (PCO2 ~ mid to high 40s) may lead to some increase in PVR, reducing pulmonary blood flow and leading to earlier recovery of heart failure [20]. Needlessly high inhaled oxygen concentrations and continued high ventilatory pressures have been shown to be associated with ventilator dependence and lung injury, so a conscious effort should be made to swiftly dial down both ventilatory pressures and inhaled oxygen as heart failure improves.
Unfortunately, most pediatricians defer intubation till the child is in extremis. In fact, there is evidence that early recourse to ventilatory support (noninvasive or invasive) improves heart failure sooner [18, 19].
Measures to reduce metabolic demand, that are again underutilized include the use of gentle sedation with midazolam infusions. Infants supported with CPAP may be sedated with low dose midazolam infusions– 1–2 mcg/kg/min [3] with or without added chloral hydrate. Very sick children with florid heart failure, who need assisted ventilation should be provided narcotic analgesia along with sedation – a typical drug combination being morphine and midazolam infusions. Those with high ventilatory requirements may also need muscle relaxation with atracurium or vecuronium infusions to reduce metabolic demand and provide appropriate ventilation. Analgesic, sedative and muscle relaxants should be administered ideally as infusions rather than boluses to provide steady state levels. Another underutilized simple modality is the use of gentle hypothermia (core temperatures 34°–35 °C). Hypothermia (core temperatures 32–35 °C) has been shown not only to reduce metabolic demand in heart failure but also to provide end organ protection [22, 23] . Hypothermia may be provided by cooling blankets, cool intravenous fluids and ice cold stomach washes. There has been substantial renewed interest in the therapeutic benefit of controlled hypothermia and targeted temperature control [23]. Hypothermia is a greatly underutilized therapeutic modality in our country. It is inexpensive, easy to implement and a treatment option worth exploring more intensively.
Infants with myocarditis and impaired ventricular function can often be extubated electively to nasopharyngeal or nasal CPAP [18]. They may be supported for prolonged periods with CPAP till their ventricular function improves or they cope better with suboptimal ventricular function. They can then be transferred out of the ICU to the step down unit or the ward on CPAP with careful non invasive monitoring . This leads to significant cost saving apart from freeing beds for fresh ICU admissions and has tremendous implications to clinical practice in our country.
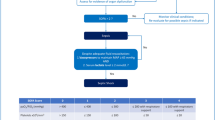
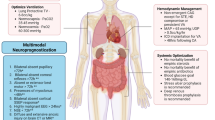
Similarly, infants with large shunts (large ventricular septal defects) or other causes of pulmonary overcirculation (dTGA with large ventricular septal defects) may also be extubated to CPAP once their heart failure improves. Thereafter, surgery can be planned during the same admission or deferred to a later date after careful cardiac evaluation. Children with acute valvar regurgitation and acute decompensated heart failure or pulmonary edema often need immediate surgery after ICU stabilization. A short period of ventilation often helps in considerable resolution of pulmonary edema (Fig. 2). Likewise infants with coarctation of aorta, anomalous origin of left coronary artery from pulmonary artery and aortic stenosis need immediate surgery or catheter intervention following ICU stabilization. Figure 3 illustrates the multimodal approach to acute deterioration of heart failure in the ICU.
Many infants with large left to right shunts and acute heart failure also have concurrent viral or bacterial chest infection and simultaneous antimicrobial or bronchodilator therapy is warranted. Nutritional support of these infants and children is best provided by gently graded hypercaloric gavage feeds via a nasogastric tube. Care must be taken to avoid acute gastric dilation while on CPAP support with periodic gastric decompression. Fluid therapy and feed volumes are titrated to clinical signs of hydration and fluid loading.
Hypercyanotic Spell or Tet Spell
Another common indication for ICU admission is the “sick cyanotic infant or hypercyanotic older child” presenting with worsening cyanosis and hyperpnea [2].
Cyanotic or hypercyanotic spells are characterized by paroxysms of hyperpnea (rapid and deep respirations), irritability, prolonged crying and increased cyanosis. If not treated in time, it may lead to profound hypoxemia, seizures, cerebrovascular accidents with neurological deficits or death. A spell occurs most often in infants less than 2-y-old, on waking up in the morning, following a crying episode or following a bath. Other triggering factors of a spell include fever, viral infections especially bronchiolitis or anemia.
The principle of acute management revolves around increasing systemic vascular resistance and reducing the pulmonary vascular resistance so that antegrade pulmonary flow is improved and right to left shunting is minimized.
So, current management of spell includes:
-
1.
Oxygen (via a face mask or hood) to reduce pulmonary vascular resistance
-
2.
Squatting position to increase systemic vascular resistance (SVR) if the infant is willing to squat ! The other option is to get the mother to hold the infant to her chest with folded knees.
-
3.
Intravenous fluids for volume repletion with crystalloid or colloid solutions if the child is dehydrated or very polycythemic.
-
4.
Beta blockers to reduce cardiac and infundibular contractility. Short acting betablockers (esmolol 25–300 mcg/kg/min or metoprolol 1–5 mcg/kg/min) infusions are currently preferred to oral or intravenous propranolol since the latter may sometimes induce profound and sustained bradycardia needing active resuscitation.
-
5.
Sedation with short acting sedatives – midazolam or lorazepam infusion to control hyperventilation. The author prefers to avoid traditional drugs like morphine due to its respiratory depressant effect which may sometimes induce profound apnea needing intubation and ventilation.
-
6.
Anemia, if present needs to be corrected.
-
7.
Occasionally, correction of metabolic acidosis with injection sodium bicarbonate 1 meq/kg may be needed. It reduces acidosis induced respiratory stimulation and pulmonary vascular resistance, improving antegrade pulmonary flow.
-
8.
Phenylephrine may be tried with utmost caution in extreme situations as they are potent vasoconstrictors which may jeopardize organ perfusion.
Many of these infants are extremely irritable, restless and agitated. Hence traditional measures like “knee chest position” are not easy to sustain. Likewise morphine often induces profound respiratory depression and is best avoided when short acting sedatives are available, today. Most infants and children in a cyanotic spell often stabilize with a combination of short acting sedatives and metoprolol infusion. It may then be possible to move the child from the ICU to a step down bed, freeing much needed ICU beds.
If the child does not improve with all these measures, high risk systemic to pulmonary artery shunt or definitive surgery needs to be done on an emergency basis.
Arrhythmias
Arrhythmias that may need ICU care include both tachyarrhythmias (Heart rate >250/min in neonates, 220/min in infants and 150/min in older children) or bradyarrhythmias [24]. Both conditions if allowed to persist can rapidly evolve into cardiac failure, secondary low output state and cardiogenic shock. Most of these arrhythmias are potentially treatable and often are seen in children with structurally normal hearts . There is an entire section on arrhythmias in this symposium, so it will not be discussed further.
Detailed algorithms are available in the American Heart Association, 2010 Guidelines for Pediatric Basic Life Support [25].
Pericardial Tamponade Needing Urgent Drainage
Occasionally, infants or children need ICU admission for large pericardial effusions causing tamponade. Pericardial effusions occur following viral infections, may be suppurative, part of heart failure or following atrial line or pacing wire removal. These children very swiftly develop a low output state along with hepatomegaly and tachycardia. A swift bedside echo confirms the diagnosis. An echo guided pericardial tap is life saving.
Heart Failure with Concurrent Chest Infections
Many infants with large “left to right shunts” and acute heart failure also have concurrent viral or bacterial chest infections and constitute a frequent indication for ICU admission. These infants need aggressive heart failure management along with simultaneous antimicrobial or bronchodilator therapy. In addition, temperature control with antipyretics 4 hourly is important to control temperature related tachycardia. Many of these infants have greatly increased work of breathing but often can be successfully managed with non invasive ventilation using judicious levels of CPAP (7–10 mm). The CPAP not only improves the heart failure but also acts as a lung recruitment strategy in chest infections improving tissue oxygenation and pulmonary hypertension. Sometimes, they remain agitated on CPAP and are unable to sustain airway pressures. In that case gentle sedation with chloral hydrate with or without midazolam infusion is often helpful. Again, caution is recommended about using excessive oxygen concentrations (FiO2) to avoid “lung flooding” and worsening of heart failure due to acute “oxygen related” reduction in pulmonary vascular resistance. A FiO2 of 0.3–0.4 is usually appropriate and sufficient with a target saturation of low to mid 90s, thus limiting pulmonary blood flow without compromising tissue oxygenation [20].
They can then be transferred out of the ICU to the step down unit or the ward on CPAP with careful non invasive monitoring. This leads again to significant cost saving apart from freeing beds for fresh ICU admissions [26].
A pre emptive attitude to infants with heart failure and chest infection often averts intubation and subsequent ventilatory dependence. Many such infants can be managed with non invasive ventilation if such support is provided early in the course of the disease. Care should be taken to avoid excessive oxygen delivery to limit pulmonary blood flow.
Cyanotic Heart Disease with Cerebrovascular Accident or Brain Abscess
Cyanotic infants following a “tet spell” may develop a cerebrovascular accident. In our country hypercyanotic polycythemic older children often develop a cerebrovascular accident or a brain abscess needing admission to the ICU. Infarcts in these children may be hypoxemic or hemorrhagic leading to significant cerebral edema. In fact, with greater awareness increasing number of cyanotics with stroke syndromes and progressive cerebral edema often need ICU care. Many of these children present with just hyperpnea and extreme irritability making initial diagnosis difficult. Sometimes they present with seizures or altered sensorium due to increased intracranial pressures. It is of utmost importance that diagnosis is timely so that neuroprotective measures may be swiftly instituted [27]. This is best done by airway control by intubation and controlled ventilation to prevent hypercarbia. Hemodynamic stability is of paramount importance to maintain adequate cerebral perfusion pressures (CPP). Euvolemia needs to be maintained with appropriate isotonic fluids. Both fluid overloading as well as dehydration should be addressed. Likewise euglycemia with appropriate glucose infusion rates should be maintained. Seizures should be aggressively managed with midazolam, phenobarbitone or levetiracetam. Fever should be controlled since fever increases cerebral metabolism. Currently, there is emerging data on the therapeutic benefits of targeted temperature control i.e., controlled hypothermia [28, 29]. Hypothermia should be carefully monitored due to attendant risks of hypothermia related infection, coagulopathy and arrhythmias in the cardiac patient.
Many cyanotic infants and children present with “stroke syndromes” and need admission to the ICU. A protocolized pre emptive approach focussed on aggressive neuroprotection along with therapeutic hypothermia is often life saving and also minimizes neuromorbidity.
These children need urgent and often serial neuroimaging (CT scans or MRI). Ideally, they also need intracranial pressure monitoring. In the event of ventriculomegaly or worsening brain edema, timely neurosurgical intervention is not only life saving but also minimizes neuromorbidity.
Many of these children do recover with good long term neurologic outcomes if timely and aggressive neuroprotection is instituted. Figure 4 illustrates the step wise approach to such children.
Neonates with Urgent Heart Disease
The next group of patients who need ICU admission are sick neonates with “urgent heart disease” i.e., those who need early or immediate intervention. These neonates typically present in a dramatic fashion with 1) profound cyanosis– dextro-Transposition of the great arteries with intact ventricular septum (dTGA.IVS), pulmonary stenosis or pulmonary atresia 2) circulatory collapse or shock (severe left sided obstructions) or 3) cyanosis with heart failure or collapse – obstructed total anomalous pulmonary venous connexion (TAPVC) [4, 7].
Neonates typically deteriorate as the ductus arteriosus begins to close, hence neonatal urgent heart disease is synonymous with duct dependent heart disease. Thus, these infants have duct dependent pulmonary circulation (pulmonary stenosis or pulmonary atresia) or duct dependent systemic circulation (critical aortic stenosis, aortic coarctation or interruption or hypoplastic left heart). The ductus also provides an important source of intercirculatory mixing in d transposition of great arteries (dTGA.IVS). Thus initial stabilization involves timely restoration of ductal circulation.
In the absence of an echo, Prostaglandin E1 (PGE1) may be commenced on the suspicion of duct dependent heart disease i.e., 1) those who fail the hyperoxia test 2) the shocky neonate in the first 2 wk of life. PGE1 is given in doses of 0.025–0.1 mcg/kg/min and due to its side effects of apnea, fever, vasodilatation, vigilant monitoring is advised. PGE1 may sometimes restore ductal circulation for few weeks after birth. This is important to know since in our country late presentation of neonatal heart disease is invariable. In some cases, the baby may not improve or may actually deteriorate with PGE1. Then the PGE1 should be ceased and if possible, an urgent review ECHO be done. If this is not feasible, the baby should be urgently transferred to a cardiac unit. The usual causes of such deterioration are 1) obstructed TAPVC 2) dTGA.IVS with restrictive patent foramen ovale (PFO) 3) Mitral atresia with restrictive PFO.
Simultaneously, basics of advanced life support should be initiated– oxygen to maintain saturations upto 85 %, a stable airway and a reliable venous access. If there is profound cyanosis or severe respiratory distress it may be prudent to intubate. Again, the goal of ventilation is to avoid over oxygenation and alkalosis with an acute reduction in PVR, so it is usual to ventilate in air [20]. Volume repletion with saline or isotonic fluids is important since the neonate has a noncompliant myocardium and also because of PGE1 induced vasodilation. Sometimes inotropy with dobutamine may be needed once volume repletion is achieved. It is important to also maintain euglycemia, normocalcemia and to exclude concurrent sepsis.
Neonates very rapidly develop uncompensated low cardiac output states due to their noncompliant myocardium and restrictive physiology, which makes them intolerant to greatly increased afterload, poorly responsive to increased preload and with a real predilection for pulmonary hypertension. They, thus, respond rapidly and profoundly to stress with drastic changes in pH, acid base status, lactate and glucose, becoming desperately unwell very quickly. It is, thus, of paramount importance to recognize a sick cardiac neonate and initiate intensive care management before the baby becomes irreversibly sick.
Neonates in our country invariably present late, hence are often sicker at presentation and many have profound end organ dysfunction on admission. Thus, the intensivist after initial stabilization also needs to carefully review all organ functions apart from the heart. Sick neonates may develop myocardial dysfunction due to protracted acidosis , ischemic bowel, varying degrees of transaminitis, renal injury and coagulopathy with bleeding. Some may also have significant neurological injury which would need to be carefully evaluated before definitive treatment is offered.
Conclusions
In summary, both NICUs and PICUs across our country now have an increasing number of sick cardiac admissions. There may be associated sepsis, endocarditis, chest infections or stroke syndromes, all of which can make intensive care challenging. It is important to note, however that with timely and speedy management, intact survival is possible in a number of sick cardiac infants and children. It is also heartening that many treatment modalities which have evoked renewed interest like therapeutic hypothermia and noninvasive ventilation are inexpensive and accessible to many treating units. Thus, despite, the ever increasing burden of heart disease in our country, a lot is eminently possible.
References
Saxena A. Congenital heart disease in India: a status report. Indian J Pediatr. 2005;72:595–8.
Yates MC, Rao PS. Pediatric cardiac emergencies. Emerg Med. 2013;3:1–7.
Rossano JW, Price JF, Nelson DP. Treatment of heart failure in infants and children : medical management. In: Nichols DG, editor. Rogers’s textbook of pediatric intensive care. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 1093–108.
Wessel DL, Fraisse A. Preoperative care of the pediatric cardiac surgical patient. In: Nichols DG, editor. Rogers’s textbook of pediatric intensive care. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 1149–58.
Hsu DT, Pearson GD. Heart failure in children part I: history, etiology, and pathophysiology. Circ Heart Fail. 2009;2:63–70.
Hsu DT, Pearson GD. Heart failure in children part II: diagnosis, treatment, and future directions. Circ Heart Fail. 2009;2:490–8.
Kumar G, Iyer PU. Neonatal cardiac emergencies. In: Choudhury P, Bagga A, Chugh K, Ramji S, Gupta P, editors. Principles of pediatric & neonatal emergencies. A publication of Indian Pediatrics. 3rd ed. New Delhi: Jaypee; 2011. p. 579–90.
Halley GC, Tibby S. Hemodynamic monitoring. In: Nichols DG, editor. Rogers’s textbook of pediatric intensive care. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 1039–63.
Carcillo JA, Kuch BA, Han YY, et al. Mortality and functional morbidity after use of PALS/APLS by community physicians. Pediatrics. 2009;124:500–8.
Hatherill M, McIntyre AG, Wattie M, Murdoch IA. Early hyperlactataemia in critically ill children. Intensive Care Med. 2000;26:314–8.
de Oliveira CF, de Oliveira DS, Gottschald AF, et al. ACCM/PALS hemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34:1065–75.
Dent CL, Nelson DP. Low cardiac output syndrome in the intensive care setting. In: Chang AC, Towbin JA, editors. Heart failure in children and young adults: from molecular mechanisms to medical and surgical strategies. 1st ed. Philadelphia: Saunders Elsevier; 2006. p. 517–34.
Kantor PF, Lougheed J, Dancea A, et al. Society guidelines presentation, diagnosis, and medical management of heart failure in children: Canadian Cardiovascular Society Guidelines. Can J Cardiol. 2013;29:1535–52.
Maiya S, Sullivan I, Allgrove J, et al. Hypocalcaemia and vitamin D deficiency: an important, but preventable, cause of life‐threatening infant heart failure. Heart. 2008;94:581–4.
Schwartz SM, Duffy JY, Pearl JM, Nelson DP. Cellular and molecular aspects of myocardial dysfunction. Crit Care Med. 2001;29:S214–9.
Schoemaker RC, van dDer Vorst MM, van Heel IR, Cohen AF, Burggraaf J; Pediatric Pharmacology Network. Development of an optimal furosemide infusion strategy in infants with modeling and simulation. Clin Pharmacol Ther. 2002;72:383–90.
Luciani GB, Nichani S, Chang AC, et al. Continuous versus intermittent furosemide infusion in critically ill infants after open heart operations. Ann Thorac Surg. 1997;64:1133–9.
Shekerdemian L. Nonpharmacologic treatment of acute heart failure. Curr Opin Pediatr. 2001;13:240–6.
Shekerdemian L. Cardiorespiratory interactions in children with heart disease. In: Nichols DG, editor. Rogers’s textbook of pediatric intensive care. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 1028–38.
Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child. 1999;80:475–80.
Marraro GA. Innovative practices of ventilatory support with pediatric patients. Pediatr Crit Care Med. 2003;4:8–20.
Deakin CD, Knight H, Edwards JC, et al. Induced hypothermia in the post operative management of refractory cardiac failure following paediatric cardiac surgery. Anesthesia. 1998;53:848–53.
Broessner G, Fischer M, Schubert G, Metzler B, Schmutzhard E. Update on therapeutic temperature management. Crit Care. 2012;16:S1–42.
Marino BS, Kaltman JR, Tanel RE. Cardiac conduction, dysrhythmias and pacing. In: Nichols DG, editor. Rogers’s textbook of pediatric intensive care. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 1126–48.
Berg MD, Schexnayder SM, Chameides L, et al. Pediatric basic life support: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Pediatrics. 2010;126:e1345–60.
Hill NS, Brennan J, Garpestad E, Nava S. Non-invasive ventilation in acute respiratory failure. Crit Care Med. 2007;35:2402–7.
Pappachan J, Kirkham F. Cerebrovascular disease and stroke. In: Nichols DG, editor. Rogers’s textbook of pediatric intensive care. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. p. 929–53.
Hemmen T, Rapp K, Raman R, et al. Phase 2/3 study of intravenous thrombolysis and hypothermia for acute treatment of ischemic stroke (ICTuS 2/3). Crit Care. 2012;16:A13.
Kollmar R, Schwab S, Staykov D. Therapeutic hypothermia decreases growth of perihemorrhagic edema and prevents critical increase of intracranial pressure in large intracerebral haemorrhage. Crit Care. 2012;16:A14.
Guarantor
PUI will act as guarantor for this paper.
Conflict of Interest
None.
Source of Funding
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iyer, P.U. Management Issues in Intensive Care Units for Infants and Children with Heart Disease. Indian J Pediatr 82, 1164–1171 (2015). https://doi.org/10.1007/s12098-015-1914-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-015-1914-0