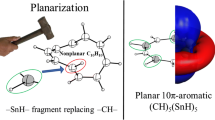
Abstract
Structural variations of different 2 π-aromatic three-membered ring systems of main group elements, especially group 14 and 13 elements as compared to the classical description of cyclopropenyl cation has been reviewed in this article. The structures of heavier analogues as well as group 13 analogues of cyclopropenyl cation showed an emergence of dramatic structural patterns which do not conform to the general norms of carbon chemistry. Isolobal analogies between the main group fragments have been efficiently used to explain the peculiarities observed in these three-membered ring systems.

Structural variations of 2π-aromatic three-membered ring systems of main group elements have been reviewed in this article. The peculiarities observed in these three-membered ring systems have been explained through the concept of isolobal analogy, diagonal relationship and pseudo 2π-aromaticity.
























Similar content being viewed by others
References
(a) Breslow R 1957 J. Am. Chem. Soc. 79 5318; (b) Breslow R and Groves J T 1970 J. Am. Chem. Soc. 92 (4) 984
(a) Walsh A D 1947 Nature 159 712; (b) Walsh A D 1949 Trans. Faraday Soc. 45 179
(a) Hückel E 1931 Zeitschrift fur Physik 70 204; (b) Hückel E 1932 Zeitschrift fur Physik 76 628; (c) Hückel E 1937 Zeitschrift fur Elektrochemie und Angewandte Physikalische Chemie 42 752
Jemmis E D, Srinivas G N, Leszczynski J, Kapp J, Korkin A A and Schleyer P v R 1995 J. Am. Chem. Soc. 117(45) 11361
(a) Rubio J and Illas F 1984 J. Mol. Struct. 110 131; (b) Srinivas N G, Kiran B and Jemmis E D 1996 J. Mol. Struct. 361 205
(a) Li X W, Penninghton W T and Robinson G H 1995 J. Am. Chem. Soc. 117 7578; (b) Li X W, Xie Y, Schreiner P R, Gripper K D, Crittendon R C, Campana C F, Schaefer H F and Robinson G H 1996 Organometallics 15 3798; (c) Xie Y, Schreiner P R, Schaefer H F, Li X W and Robinson G H 1996 J. Am. Chem. Soc. 118 10635
Srinivas G N, Jemmis E D, Korkin A A and Schleyer P V R 1999 J. Phys. Chem. A 103 11034
Eisch J J, Shatii B and Rheingold A L 1987 J. Am. Chem. Soc. 109 2526
Balucani N, Asvany O, Lee Y T and Kaiser R I 2000 J. Am. Chem. Soc. 122 11234
Krogh-Jespersen K, Cremer D, Dill J D, Pople J A and Schleyer P V R 1981 J. Am. Chem. Soc. 103 2589
Srinivas R, Sulzle D and Schwarz H 1990 J. Am. Chem. Soc. 112 8334
Korkin A A, Schleyer P V R, Arx U V and Keese R 1995 Struc. chem. 6 225
Wehrmann R, Meyer H and Bemdt A 1985 Angew. Chem. Int. Ed. Engl. 24 788
(a) Korkin A A, Schleyer P v R and McKee M L 1995 Inorg. Chem. 34 961; (b) Schleyer P v R, Subramanian G and Dansfeld A 1996 J. Am. Chem. Soc. 118 9988; (c) McKee M L, Buhl M, Charkin O P and Schleyer P v R 1993 Inorg. Chem. 32 4549; (d) Krempp M, Damrauer R, DePuy C H and Keheyan Y 1994 J. Am. Chem. Soc. 116 3629; (e) Glukhovtsev M N, Schleyer P v R, Hommes N J R V E, Carn-Eiro J W D M and Koch W 1993 Comput. Chem 14 285
(a) Jemmis E D, Subramanian G and Srinivas G N 1992 J. Am. Chem. Soc. 114 7939; (b) Skancke A and Liebman J F 1993 J. Mol. Struct.(THEOCHEM) 280 75; (c) McKee M L 1995 J. Am. Chem. Soc. 117 8001; (d) McKee M L 1999 Inorg. Chem. 38 321
Schulenberg N, Wadepohl H and Himmel H-J 2011 Angew. Chem. Int. Ed. 50 10444
Jemmis E D and Subramanian G 1995 Inorg. Chem. 34 6559
Arunan E and Mandal P K 2001 J. Chem. Phys. 114 3880
Hari Krishna Reddy K and Jemmis E D 2013 Dalton Trans. 42 10633
Braunschweig H, Dewhurst R D, Hammond K, Mies J, Radacki K and Vargas A 2012 Science 336 1420
Braunschweig H, Damme A, Dewhurst R D and Vargas A 2013 Nat. Chem. 5 115
(a) Jemmis E D, Prasad B V, Prasad P V A, Tsuzuki S and Tanabe K 1990 Proc. Ind. Acad. Sci. (Chem. Sci.) 102 107; (b) Jemmis E D, Prasad B V, Tsuzuki S and Tanabe K 1990 J. Phys. Chem. 94 5530
Subramanian G and Jemmis E D 1992 Chem. Phys. Lett. 200 567
Subramanian G, Jemmis E D and Prasad B V 1994 Chem. Phys. Lett. 217 296
Srinivas G N, Anoop A, Jemmis E D, Hamilton T P, Lammertsma K, Leszczynski J and Schaefer H F 2003 J. Am. Chem. Soc. 125 16397
Srinivas G N, Kiran B and Jemmis E D 1996 J. Mol. Stru. THEOCHEM 361 205
(a) Jemmis E D and Parameswaran P 2007 Chem. Eur. J. 13 2622; (b) Mallick D, Parameswaran P and Jemmis E D 2008 J. Phys. Chem. A 112 3080
(a) Eggerding D and West R 1975 J. Am. Chem. Soc. 97 207; (b) Eggerding D and West R 1976 J. Am. Chem. Soc. 98 3641; (c) Summerscales O T, Cloke F G N, Hitchcock P B, Green J C and Hazari N 2006 Science 311 829
Priyakumari C P and Jemmis E D 2013 J. Am. Chem. Soc. 135 16026
Acknowledgements
We thank the Supercomputer Education and Research Centre (SERC), Indian Institute of Science, Bangalore and the Centre for Modelling Simulation and Design (CMSD), University of Hyderabad for computational facilities. EDJ thanks the Department of Science and Technology (DST) for J C Bose Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MALLICK, D., JEMMIS, E.D. Structural variations in aromatic 2π-electron three-membered rings of the main group elements. J Chem Sci 127, 183–196 (2015). https://doi.org/10.1007/s12039-015-0777-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0777-2