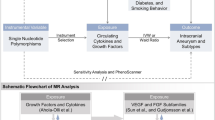
Abstract
Subarachnoid hemorrhage (SAH) is a neurological emergency that can lead to fatal outcomes. It occurs when bleeding happens in the subarachnoid space, a small gap between the arachnoid and pia mater. This condition results from the rupture of diseased or damaged blood vessels at the brain’s base or surface. This study combined various omics approaches with Mendelian randomization analysis, including MR-IVW, MR Egger, MR weight median, and MR weight mode, to generate preliminary results. It also employed reverse Mendelian randomization, treating SAH as the exposure. Finally, a meta-analysis was conducted to summarize these findings. The study found positive correlations between SAH and both GBPA-Pyridoxal 5 phosphate biosynthesis I (OR=1.48, 95% CI, 1.04–2.12) and GBPA-glucose biosynthesis I (OR=0.68, 95% CI, 0.52–0.90). Increased levels of urokinase-type plasma activator were also associated with SAH (OR=1.17, 95% CI, 1.04–1.32). Associations were observed with SAH for CD80 on CD62L+ plasmacytoid dendritic cells, CD80 on plasmacytoid dendritic cells, CD123 on CD62L+ plasmacytoid dendritic cells, and SSC-A on plasmacytoid dendritic cells. This study, through Mendelian randomization and meta-analysis, established links between SAH and four inflammatory cells, one inflammatory protein, and two gut microbiota-related pathways. These findings suggest potential treatment targets for SAH, highlighting the importance of modulating gut microbiota and utilizing anti-inflammatory drugs in its management.





Similar content being viewed by others
Data Availability
The datasets generated and/or analyzed during the current study are available in the in this website (https://pheweb.org/UKB-SAIGE/) and IEU OpenGWAS (https://gwas.mrcieu.ac.uk/), and Phenoscanner (http://www.phenoscanner.medschl.cam.ac.uk/).
Abbreviations
- SAH:
-
Subarachnoid hemorrhage
- MR:
-
Mendelian randomization
- IVW:
-
Inverse variance weighted
- SNPs:
-
Single nucleotide polymorphism
- GBPA:
-
Gut bacterial pathway abundance
- ICH:
-
Intracerebral Hemorrhage
- TNF-α:
-
Tumor Necrosis Factor-alpha
- IL-6:
-
Interleukin 6
- IL-2:
-
Interleukin-2
- NKs:
-
Natural killer cells
- Tregs:
-
Tegulatory T cells
References
Muehlschlegel S (2018) Subarachnoid Hemorrhage. Continuum (Minneap Minn) 24:1623–1657
Tao M, Mao J, Bao Y, Liu F, Mai Y, Guan S, Luo S, Huang Y et al (2023) A blood-responsive AIE bioprobe for the ultrasensitive detection and assessment of subarachnoid hemorrhage. Adv Sci (Weinh) 10:e2205435
Tawk RG, Hasan TF, D'Souza CE, Peel JB, Freeman WD (2021) Diagnosis and treatment of unruptured intracranial aneurysms and aneurysmal subarachnoid hemorrhage. Mayo Clin Proc 96:1970–2000
D'Amato SA, Chang TR (2023) Advances in intracranial hemorrhage: subarachnoid hemorrhage and intracerebral hemorrhage. Crit Care Clin 39:71–85
Chung DY, Abdalkader M, Nguyen TN (2021) Aneurysmal subarachnoid hemorrhage. Neurol Clin 39:419–442
Luo C, Yao J, Bi H, Li Z, Li J, Xue G, Li K, Zhang S et al (2022) Clinical value of inflammatory cytokines in patients with aneurysmal subarachnoid hemorrhage. Clin Interv Aging 17:615–626
Gao S, Zhou L, Lu J, Fang Y, Wu H, Xu W, Pan Y, Wang J et al (2022) Cepharanthine attenuates early brain injury after subarachnoid hemorrhage in mice via inhibiting 15-Lipoxygenase-1-mediated microglia and endothelial cell ferroptosis. Oxid Med Cell Longev 2022:4295208
Muhammad S, Hanggi D (2021) Inflammation and anti-inflammatory targets after aneurysmal subarachnoid hemorrhage. Int J Mol Sci 22(14):7355. https://doi.org/10.3390/ijms22147355
Tian Q, Guo Y, Feng S, Liu C, He P, Wang J, Han W, Yang C et al (2022) Inhibition of CCR2 attenuates neuroinflammation and neuronal apoptosis after subarachnoid hemorrhage through the PI3K/Akt pathway. J Neuroinflammation 19:312
Bjerkne Wenneberg S, Odenstedt Herges H, Svedin P, Mallard C, Karlsson T, Adiels M, Naredi S et al (2021) Association between inflammatory response and outcome after subarachnoid haemorrhage. Acta Neurol Scand 143:195–205
Wang XY, Wu F, Zhan RY, Zhou HJ (2022) Inflammatory role of microglia in brain injury caused by subarachnoid hemorrhage. Front Cell Neurosci 16:956185
Cao Y, Li Y, He C, Yan F, Li JR, Xu HZ, Zhuang JF, Zhou H et al (2021) Selective ferroptosis inhibitor Liproxstatin-1 attenuates neurological deficits and neuroinflammation after subarachnoid hemorrhage. Neurosci Bull 37:535–549
Fragata I, Bustamante A, Penalba A, Ferreira P, Nunes AP, Canhao P, Montaner J (2020) TNF-R1 correlates with cerebral perfusion and acute ischemia following subarachnoid hemorrhage. Neurocrit Care 33:679–687
Li K, Barras CD, Chandra RV, Kok HK, Maingard JT, Carter NS, Russell JH, Lai L et al (2019) A review of the management of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. World Neurosurg 126:513–527
Solar P, Zamani A, Lakatosova K, Joukal M (2022) The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: molecular events and potential treatments. Fluids Barriers CNS 19:29
Su J, Wang M, Yan Y, Ju S, Chen J, Wu X (2019) Increased REDD1 facilitates neuronal damage after subarachnoid hemorrhage. Neurochem Int 128:14–20
Shikata F, Shimada K, Sato H, Ikedo T, Kuwabara A, Furukawa H, Korai M, Kotoda M et al (2019) Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension 73:491–496
Zhang P, Wang R, Qu Y, Guo ZN, Yang Y (2023) Gut microbiota-derived metabolite trimethylamine-N-oxide and stroke outcome: a systematic review. Front Mol Neurosci 16:1165398
Al Bander Z, Nitert MD, Mousa A, Naderpoor N (2020) The gut microbiota and inflammation: an overview. Int J Environ Res Public Health 17(20):7618. https://doi.org/10.3390/ijerph17207618
Cai J, Sun L, Gonzalez FJ (2022) Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 30:289–300
Scheithauer TPM, Rampanelli E, Nieuwdorp M, Vallance BA, Verchere CB, van Raalte DH, Herrema H (2020) Gut microbiota as a trigger for metabolic inflammation in obesity and type 2 diabetes. Front Immunol 11:571731
Bowden J, Holmes MV (2019) Meta-analysis and Mendelian randomization: A review. Res Synth Methods 10:486–496
Lopera-Maya EA, Kurilshikov A, van der Graaf A, Hu S, Andreu-Sanchez S, Chen L, Vila AV, Gacesa R et al (2022) Effect of host genetics on the gut microbiome in 7,738 participants of the Dutch Microbiome Project. Nat Genet 54:143–151
Zhao JH, Stacey D, Eriksson N, Macdonald-Dunlop E, Hedman AK, Kalnapenkis A, Enroth S, Cozzetto D et al (2023) Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol 24:1540–1551
Chen MH, Raffield LM, Mousas A, Sakaue S, Huffman JE, Moscati A, Trivedi B, Jiang T et al (2020) Trans-ethnic and ancestry-specific blood-cell genetics in 746,667 individuals from 5 global populations. Cell 182(1198-1213):e1114
Naidech AM (2011) Intracranial hemorrhage. Am J Respir Crit Care Med 184:998–1006
Roethlisberger M, Achermann R, Bawarjan S, Stienen MN, Fung C, D'Alonzo D, Maldaner N, Ferrari A et al (2018) Swiss SOSSG: Predictors of occurrence and anatomic distribution of multiple aneurysms in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg 111:e199–e205
Corovic A, Kelly S, Markus HS (2018) Cerebral amyloid angiopathy associated with inflammation: A systematic review of clinical and imaging features and outcome. Int J Stroke 13:257–267
Weinstock MJ, Uhlmann EJ, Zwicker JI (2016) Intracranial hemorrhage in cancer patients treated with anticoagulation. Thromb Res 140(Suppl 1):S60–S65
Ziai WC, Thompson CB, Mayo S, McBee N, Freeman WD, Dlugash R, Ullman N, Hao Y et al (2019) Clot lysis: evaluating accelerated resolution of intraventricular hemorrhage i: intracranial hypertension and cerebral perfusion pressure insults in adult hypertensive intraventricular hemorrhage: occurrence and associations with outcome. Crit Care Med 47:1125–1134
Lazzaro MA, Ouyang B, Chen M (2012) The role of circle of Willis anomalies in cerebral aneurysm rupture. J Neurointerv Surg 4:22–26
Lindner SH, Bor AS, Rinkel GJ (2010) Differences in risk factors according to the site of intracranial aneurysms. J Neurol Neurosurg Psychiatry 81:116–118
Korja M, Kaprio J (2016) Controversies in epidemiology of intracranial aneurysms and SAH. Nat Rev Neurol 12:50–55
He M, Wang W, He Q, Dai H, Han J, Cui W (2023) Genetic causal association between the gut microbiome and intracranial aneurysm and subarachnoid hemorrhage: a two-sample Mendelian randomization study. Neurol Ther 12:1695–1707
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140:918–934
Schneider UC, Xu R, Vajkoczy P (2018) Inflammatory events following subarachnoid hemorrhage (sah). Curr neuropharmacol 16:1385–1395
Penn DL, Witte SR, Komotar RJ, Sander Connolly E (2015) Pathological mechanisms underlying aneurysmal subarachnoid haemorrhage and vasospasm. J Clin Neurosci 22:1–5
Zhang ZH, Han YL, Wang CX, Zhou CH, Wu LY, Zhang HS, Chen Q, Fan JM et al (2016) The effect of subarachnoid erythrocyte lysate on brain injury: a preliminary study. Biosci Rep 36. https://doi.org/10.1042/BSR20160100
Gris T, Laplante P, Thebault P, Cayrol R, Najjar A, Joannette-Pilon B, Brillant-Marquis F, Magro E et al (2019) Canadian Critical Care Translational Biology G: Innate immunity activation in the early brain injury period following subarachnoid hemorrhage. J Neuroinflammation 16:253
McMahon CJ, Hopkins S, Vail A, King AT, Smith D, Illingworth KJ, Clark S, Rothwell NJ et al (2013) Inflammation as a predictor for delayed cerebral ischemia after aneurysmal subarachnoid haemorrhage. J Neurointerv Surg 5:512–517
Zhang J, Xu X, Zhou D, Li H, You W, Wang Z, Chen G (2015) Possible role of Raf-1 kinase in the development of cerebral vasospasm and early brain injury after experimental subarachnoid hemorrhage in rats. Mol Neurobiol 52:1527–1539
Nakura T, Osuka K, Inukai T, Takagi T, Takayasu M (2011) Soluble gp130 regulatess interleukin-6 in cerebrospinal fluid after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 82:952–954
Schumacher N, Yan K, Gandrass M, Muller M, Krisp C, Hasler R, Carambia A, Nofer JR et al (2021) Cell-autonomous hepatocyte-specific GP130 signaling is sufficient to trigger a robust innate immune response in mice. J Hepatol 74:407–418
Burton MD, Johnson RW (2012) Interleukin-6 trans-signaling in the senescent mouse brain is involved in infection-related deficits in contextual fear conditioning. Brain Behav Immun 26:732–738
Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA et al (2008) IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol 181:2189–2195
Bonomi A, Veglia F, Baldassarre D, Strawbridge RJ, Golabkesh Z, Sennblad B, Leander K, Smit AJ, et al., on behalf of the Isg: Analysis of the genetic variants associated with circulating levels of sgp130. Results from the IMPROVE study. Genes Immun 2020;21:100-108.
Elliott EI, Sutterwala FS (2015) Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev 265:35–52
Gaidt MM, Hornung V (2018) The NLRP3 inflammasome renders cell death pro-inflammatory. J Mol Biol 430:133–141
Li JR, Xu HZ, Nie S, Peng YC, Fan LF, Wang ZJ, Wu C, Yan F et al (2017) Fluoxetine-enhanced autophagy ameliorates early brain injury via inhibition of NLRP3 inflammasome activation following subrachnoid hemorrhage in rats. J Neuroinflammation 14:186
Xu Q, Wang M, Guo H, Liu H, Zhang G, Xu C, Chen H (2021) Emodin alleviates severe acute pancreatitis-associated acute lung injury by inhibiting the cold-inducible RNA-binding protein (CIRP)-mediated activation of the NLRP3/IL-1beta/CXCL1 signaling. Front Pharmacol 12:655372
Xu X, Zhang L, Ye X, Hao Q, Zhang T, Cui G, Yu M (2018) Nrf2/ARE pathway inhibits ROS-induced NLRP3 inflammasome activation in BV2 cells after cerebral ischemia reperfusion. Inflamm Res 67:57–65
Chen J, Chen G, Li J, Qian C, Mo H, Gu C, Yan F, Yan W et al (2014) Melatonin attenuates inflammatory response-induced brain edema in early brain injury following a subarachnoid hemorrhage: a possible role for the regulation of pro-inflammatory cytokines. J Pineal Res 57:340–347
Liu H, Yang M, Pan L, Liu P, Ma L (2016) Hyperbaric oxygen intervention modulates early brain injury after experimental subarachnoid hemorrhage in rats: possible involvement of TLR4/NF-x03BA B-mediated signaling pathway. Cell Physiol Biochem 38:2323–2336
Zhou Y, Jiang Y, Peng Y, Zhang M (2017) The quantitative and functional changes of postoperative peripheral blood immune cell subsets relate to prognosis of patients with subarachnoid hemorrhage: a preliminary study. World Neurosurg 108:206–215
Ramagopalan SV, Pakpoor J, Seminog O, Goldacre R, Graham L, Goldacre MJ (2013) Risk of subarachnoid haemorrhage in people admitted to hospital with selected immune-mediated diseases: record-linkage studies. BMC Neurol 13:176
Sakaguchi S, Miyara M, Costantino CM, Hafler DA (2010) FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10:490–500
Dong G, Li C, Hu Q, Wang Y, Sun J, Gao F, Yang M, Sun B et al (2021) Low-dose IL-2 treatment affords protection against subarachnoid hemorrhage injury by expanding peripheral regulatory T cells. ACS Chem Neurosci 12:430–440
Mirlekar B, Patil S, Bopanna R, Chattopadhyay S (2015) MAR binding protein SMAR1 favors IL-10 mediated regulatory T cell function in acute colitis. Biochem Biophys Res Commun 464:647–653
Saand AR, Yu F, Chen J, Chou SH (2019) Systemic inflammation in hemorrhagic strokes - A novel neurological sign and therapeutic target? J Cereb Blood Flow Metab 39:959–988
Stern MJ, Gorman PA, Kaslow L (1983) The group counseling v exercise therapy study. A controlled intervention with subjects following myocardial infarction. Arch Intern Med 143:1719–1725
Acknowledgements
We want to acknowledge the participants and investigators of the UK Biobank study.
Author information
Authors and Affiliations
Contributions
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yan, C., Li, Y. Causal Relationships Between Gut Microbiota, Inflammatory Cells/Proteins, and Subarachnoid Hemorrhage: A Multi-omics Bidirectional Mendelian Randomization Study and Meta-analysis. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04101-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04101-y