Abstract
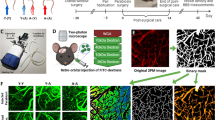
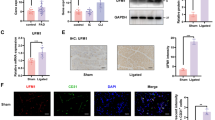
Aging is one of the key mechanisms of vascular dysfunction and contributes to the initiation and progression of ischemic stroke (IS). Our previous study demonstrated that ACE2 priming enhanced the protective effects of exosomes derived from endothelial progenitor cells (EPC-EXs) on hypoxia-induced injury in aging endothelial cells (ECs). Here, we aimed to investigate whether ACE2-enriched EPC-EXs (ACE2-EPC-EXs) could attenuate brain ischemic injury by inhibiting cerebral EC damage through their carried miR-17-5p and the underlying molecular mechanisms. The enriched miRs in ACE2-EPC-EXs were screened using the miR sequencing method. EPC-EXs, ACE2-EPC-EXs, and ACE2-EPC-EXs with miR-17-5p deficiency (ACE2-EPC-EXsantagomiR-17-5p) were administered to transient middle cerebral artery occlusion (tMCAO)-operated aged mice or coincubated with hypoxia/reoxygenation (H/R)-treated aging ECs. The results showed that (1) the level of brain EPC-EXs and their carried ACE2 were significantly decreased in aged mice compared to in young mice, and (2) after tMCAO, aged mice displayed increases in brain cell senescence, infarct volume, and neurological deficit score (NDS) and a decrease in cerebral blood flow (CBF). (3) Compared with EPC-EXs, ACE2-EPC-EXs were enriched with miR-17-5p and more effective in increasing ACE2 and miR-17-5p expression in cerebral microvessels, accompanied by obvious increases in cerebral microvascular density (cMVD) and cerebral blood flow (CBF) and decreases in brain cell senescence, infarct volume, neurological deficit score (NDS), cerebral EC ROS production, and apoptosis in tMCAO-operated aged mice. Moreover, silencing of miR-17-5p partially abolished the beneficial effects of ACE2-EPC-EXs. (4) In H/R-treated aging ECs, ACE2-EPC-EXs were more effective than EPC-EXs in decreasing cell senescence, ROS production, and apoptosis and increasing cell viability and tube formation. In a mechanistic study, ACE2-EPC-EXs more effectively inhibited PTEN protein expression and increased the phosphorylation of PI3K and Akt, which were partially abolished by miR-17-5p knockdown. Altogether, our data suggest that ACE-EPC-EXs have better protective effects on ameliorating aged IS mouse brain neurovascular injury by inhibiting cell senescence, EC oxidative stress, apoptosis, and dysfunction by activating the miR-17-5p/PTEN/PI3K/Akt signaling pathway.








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Collins C, Tzima E (2011) Hemodynamic forces in endothelial dysfunction and vascular aging. Exp Gerontol 46(2-3):185–188. https://doi.org/10.1016/j.exger.2010.09.010
Dikalov S (2011) Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51(7):1289–1301. https://doi.org/10.1016/j.freeradbiomed.2011.06.033
Zhang C, Wang J, Ma X, Wang W, Zhao B, Chen Y, Chen C, Bihl JC (2018) ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J Cell Mol Med 22(3):1873–1882. https://doi.org/10.1111/jcmm.13471
Roy-O'Reilly M, McCullough LD (2018) Age and sex are critical factors in ischemic stroke pathology. Endocrinology 159(8):3120–3131. https://doi.org/10.1210/en.2018-00465
Loiola RA, Garcia-Gabilondo M, Grayston A, Bugno P, Kowalska A, Duban-Deweer S, Rizzi E, Hachani J, et al. (2021) Secretome of endothelial progenitor cells from stroke patients promotes endothelial barrier tightness and protects against hypoxia-induced vascular leakage. Stem Cell Res Ther 12(1):552. https://doi.org/10.1186/s13287-021-02608-y
Pan Q, Zheng J, Du D, Liao X, Ma C, Yang Y, Chen Y, Zhong W, et al. (2018) MicroRNA-126 Priming enhances functions of endothelial progenitor cells under physiological and hypoxic conditions and their therapeutic efficacy in cerebral ischemic damage. Stem Cells Int 2018:2912347. https://doi.org/10.1155/2018/2912347
Wang J, Chen S, Zhang W, Chen Y, Bihl JC (2020) Exosomes from miRNA-126-modified endothelial progenitor cells alleviate brain injury and promote functional recovery after stroke. CNS Neurosci Ther 26(12):1255–1265. https://doi.org/10.1111/cns.13455
Xing Z, Zhao C, Liu H, Fan Y (2020) Endothelial Progenitor cell-derived extracellular vesicles: a novel candidate for regenerative medicine and disease treatment. Adv Healthc Mater 9(12):e2000255. https://doi.org/10.1002/adhm.202000255
de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BW (2012) Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 1:18396. https://doi.org/10.3402/jev.v1i0.18396
Ke X, Yang R, Wu F, Wang X, Liang J, Hu X, Hu C (2021) Exosomal miR-218-5p/miR-363-3p from endothelial progenitor cells ameliorate myocardial infarction by targeting the p53/JMY signaling pathway. Oxid Med Cell Longev 2021:5529430. https://doi.org/10.1155/2021/5529430
Pena Silva RA, Chu Y, Miller JD, Mitchell IJ, Penninger JM, Faraci FM, Heistad DD (2012) Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke 43(12):3358–3363. https://doi.org/10.1161/STROKEAHA.112.667063
Zheng JL, Li GZ, Chen SZ, Wang JJ, Olson JE, Xia HJ, Lazartigues E, Zhu YL, et al. (2014) Angiotensin converting enzyme 2/Ang-(1-7)/mas axis protects brain from ischemic injury with a tendency of age-dependence. CNS Neurosci Ther 20(5):452–459. https://doi.org/10.1111/cns.12233
Ma X, Wang Y, Shi Y, Li S, Liu J, Li X, Zhong W, Pan Q (2022) Exosomal miR-132-3p from mesenchymal stromal cells improves synaptic dysfunction and cognitive decline in vascular dementia. Stem Cell Res Ther 13(1):315. https://doi.org/10.1186/s13287-022-02995-w
Bao H, Gao F, Xie G, Liu Z (2015) Angiotensin-converting enzyme 2 inhibits apoptosis of pulmonary endothelial cells during acute lung injury through suppressing MiR-4262. Cell Physiol Biochem 37(2):759–767. https://doi.org/10.1159/000430393
Ning H, Zhang L, Zhu B, Zhou X, Zhang T, Ma T (2022) TARBP2-stablized SNHG7 regulates blood-brain barrier permeability by acting as a competing endogenous RNA to miR-17-5p/NFATC3 in Abeta-microenvironment. Cell Death Dis 13(5):457. https://doi.org/10.1038/s41419-022-04920-8
Hu G, Xia Y, Zhang J, Chen Y, Yuan J, Niu X, Zhao B, Li Q, et al. (2020) ESC-sEVs rejuvenate senescent hippocampal nscs by activating lysosomes to improve cognitive dysfunction in vascular dementia. Adv Sci (Weinh) 7(10):1903330. https://doi.org/10.1002/advs.201903330
Piscopo P, Grasso M, Puopolo M, D'Acunto E, Talarico G, Crestini A, Gasparini M, Campopiano R, et al. (2018) Circulating miR-127-3p as a potential biomarker for differential diagnosis in frontotemporal dementia. J Alzheimers Dis 65(2):455–464. https://doi.org/10.3233/JAD-180364
Ren X, Jing YX, Zhou ZW, Yang QM (2022) MiR-17-5p inhibits cerebral hypoxia/reoxygenationinjury by targeting PTEN through regulation of PI3K/AKT/mTOR signaling pathway. Int J Neurosci 132(2):192–200. https://doi.org/10.1080/00207454.2020.1806836
Halurkar MS, Wang J, Chen S, Bihl JC (2022) EPC-EXs improve astrocyte survival and oxidative stress through different uptaking pathways in diabetic hypoxia condition. Stem Cell Res Ther 13(1):91. https://doi.org/10.1186/s13287-022-02766-7
Sakamuri S, Sure VN, Kolli L, Liu N, Evans WR, Sperling JA, Busija DW, Wang X, et al. (2022) Glycolytic and oxidative phosphorylation defects precede the development of senescence in primary human brain microvascular endothelial cells. Geroscience. 4:1975–1994. https://doi.org/10.1007/s11357-022-00550-2
Zhang H, Pan Q, Xie Z, Chen Y, Wang J, Bihl J, Zhong W, Chen Y, et al. (2020) Implication of microRNA503 in brain endothelial cell function and ischemic stroke. Transl Stroke Res 11(5):1148–1164. https://doi.org/10.1007/s12975-020-00794-0
Ma X, Zhao J, Li S, Wang Y, Liu J, Shi Y, Liu J, Chen Y, et al. (2022) Rab27a-dependent exosomes protect against cerebral ischemic injury by reducing endothelial oxidative stress and apoptosis. CNS Neurosci Ther. 10:1596–1612. https://doi.org/10.1111/cns.13902
Wang J, Zhong Y, Ma X, Xiao X, Cheng C, Chen Y, Iwuchukwu I, Gaines KJ, et al. (2016) Analyses of endothelial cells and endothelial progenitor cells released microvesicles by using microbead and Q-dot based nanoparticle tracking analysis. Sci Rep 6:24679. https://doi.org/10.1038/srep24679
Wang W, Ma X, Han J, Zhou M, Ren H, Pan Q, Zheng C, et al. (2016) Neuroprotective effect of scutellarin on ischemic cerebral injury by down-regulating the expression of angiotensin-converting enzyme and AT1 receptor. PLoS One 11(1):e0146197. https://doi.org/10.1371/journal.pone.0146197
Wang J, Chen S, Ma X, Cheng C, Xiao X, Chen J, Liu S, Zhao B, et al. (2013) Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxid Med Cell Longev 2013:572729. https://doi.org/10.1155/2013/572729
Ma X, Zhao J, Li S, Wang Y, Liu J, Shi Y, Liu J, Chen Y, et al. (2022) Rab27a-dependent exosomes protect against cerebral ischemic injury by reducing endothelial oxidative stress and apoptosis. CNS Neurosci Ther 28(10):1596–1612. https://doi.org/10.1111/cns.13902
Tian Y, Li X, Bai C, Yang Z, Zhang L, Luo J (2020) MiR-17-5p promotes the endothelialization of endothelial progenitor cells to facilitate the vascular repair of aneurysm by regulating PTEN-mediated PI3K/AKT/VEGFA pathway. Cell Cycle 19(24):3608–3621. https://doi.org/10.1080/15384101.2020.1857958
Pan Q, Liao X, Liu H, Wang Y, Chen Y, Zhao B, Lazartigues E, Yang Y, et al. (2017) MicroRNA-125a-5p alleviates the deleterious effects of ox-LDL on multiple functions of human brain microvessel endothelial cells. Am J Physiol Cell Physiol 312(2):C119–C130. https://doi.org/10.1152/ajpcell.00296.2016
Pan Q, Kuang X, Cai S, Wang X, Du D, Wang J, Wang Y, Chen Y, et al. (2020) miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res Ther 11(1):260. https://doi.org/10.1186/s13287-020-01761-0
Li Y, Wang J, Chen S, Wu P, Xu S, Wang C, Shi H, Bihl J (2020) miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res Ther 11(1):330. https://doi.org/10.1186/s13287-020-01836-y
Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C (2005) Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol 45(9):1441–1448. https://doi.org/10.1016/j.jacc.2004.12.074
Keymel S, Kalka C, Rassaf T, Yeghiazarians Y, Kelm M, Heiss C (2008) Impaired endothelial progenitor cell function predicts age-dependent carotid intimal thickening. Basic Res Cardiol 103(6):582–586. https://doi.org/10.1007/s00395-008-0742-z
Powers WJ (2020) Acute ischemic stroke. N Engl J Med 383(3):252–260. https://doi.org/10.1056/NEJMcp1917030
Chen J, Xiao X, Chen S, Zhang C, Chen J, Yi D, Shenoy V, Raizada MK, et al. (2013) Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension 61(3):681–689. https://doi.org/10.1161/HYPERTENSIONAHA.111.00202
Ma X, Liao X, Liu J, Wang Y, Wang X, Chen Y, Yin X, Pan Q (2022) Circulating endothelial microvesicles and their carried miR-125a-5p: potential biomarkers for ischaemic stroke. Stroke Vasc Neurol.:svn-2021. https://doi.org/10.1136/svn-2021-001476
Pham TP, Bink DI, Stanicek L, van Bergen A, van Leeuwen E, Tran Y, Matic L, Hedin U, et al. (2020) Long non-coding RNA aerrie controls DNA damage repair via YBX1 to maintain endothelial cell function. Front Cell Dev Biol 8:619079. https://doi.org/10.3389/fcell.2020.619079
Zagrean AM, Hermann DM, Opris I, Zagrean L, Popa-Wagner A (2018) Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. Therapeutic Implications. Front Neurosci 12:811. https://doi.org/10.3389/fnins.2018.00811
Del Mar R-CL, Yanes-Diaz J, de Lucas B, Riestra-Ayora JI, Madrid-Garcia R, Sanz-Fernandez R, Sanchez-Rodriguez C (2022) Preventive effect of cocoa flavonoids via suppression of oxidative stress-induced apoptosis in auditory senescent cells. Antioxidants (Basel) 11(8). https://doi.org/10.3390/antiox11081450
Hu H, Wang B, Jiang C, Li R, Zhao J (2019) Endothelial progenitor cell-derived exosomes facilitate vascular endothelial cell repair through shuttling miR-21-5p to modulate Thrombospondin-1 expression. Clin Sci (Lond) 133(14):1629–1644. https://doi.org/10.1042/CS20190188
Angulski ABB, Capriglione LGA, Barchiki F, Brofman P, Stimamiglio MA, Senegaglia AC, Correa A (2019) Systemic infusion of expanded CD133(+) cells and expanded CD133(+) cell-derived EVs for the treatment of ischemic cardiomyopathy in a rat model of AMI. Stem Cells Int 2019:4802578. https://doi.org/10.1155/2019/4802578
Cantaluppi V, Gatti S, Medica D, Figliolini F, Bruno S, Deregibus MC, Sordi A, Biancone L, et al. (2012) Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 82(4):412–427. https://doi.org/10.1038/ki.2012.105
Huang R, Cheng T, Lai X (2022) Mechanism of ischemic brain injury repair by endothelial progenitor cell-derived exosomes. Mol Med Rep 26(2). https://doi.org/10.3892/mmr.2022.12785
Liu P, Zhang Q, Mi J, Wang S, Xu Q, Zhuang D, Chen W, Liu C, Zhang L, t al. (2022) Exosomes derived from stem cells of human deciduous exfoliated teeth inhibit angiogenesis in vivo and in vitro via the transfer of miR-100-5p and miR-1246. Stem Cell Res Ther 13(1):89. https://doi.org/10.1186/s13287-022-02764-9
Chiba T, Cerqueira DM, Li Y, Bodnar AJ, Mukherjee E, Pfister K, Phua YL, et al. (2021) Endothelial-derived miR-17 approximately 92 promotes angiogenesis to protect against renal ischemia-reperfusion injury. J Am Soc Nephrol 32(3):553–562. https://doi.org/10.1681/ASN.2020050717
Du L, Jiang Y, Sun Y (2021) Astrocyte-derived exosomes carry microRNA-17-5p to protect neonatal rats from hypoxic-ischemic brain damage via inhibiting BNIP-2 expression. Neurotoxicology 83:28–39. https://doi.org/10.1016/j.neuro.2020.12.006
Xin H, Liu Z, Buller B, Li Y, Golembieski W, Gan X, Wang F, Lu M, et al. (2021) MiR-17-92 enriched exosomes derived from multipotent mesenchymal stromal cells enhance axon-myelin remodeling and motor electrophysiological recovery after stroke. J Cereb Blood Flow Metab 41(5):1131–1144. https://doi.org/10.1177/0271678X20950489
Li W, Huang R, Chen Z, Yan LJ, Simpkins JW, Yang SH (2014) PTEN degradation after ischemic stroke: a double-edged sword. Neuroscience 274:153–161. https://doi.org/10.1016/j.neuroscience.2014.05.027
Zhang R, Ma X, Jiang L, Xia W, Li H, Zhao N, Cui X, Zhang N, et al. (2021) Decreased lncRNA SNHG16 accelerates oxidative stress induced pathological angiogenesis in human retinal microvascular endothelial cells by regulating miR-195/mfn2 axis. Curr Pharm Des 27(27):3047–3060. https://doi.org/10.2174/1381612827666210202141541
Ren W, Huang C, Chu H, Tang Y, Yang X (2021) Peptide5 attenuates rtPA related brain microvascular endothelial cells reperfusion injury via the Wnt/beta-catenin signalling pathway. Curr Neurovasc Res 18(2):219–226. https://doi.org/10.2174/1567202618666210809115305
Author Contribution
QP, YW, JL, XJ, YS, SL, YC, and XM performed experiments; QP, YW, and XM wrote the manuscript; QP, YW, JL, ZX, XJ, and XM contributed to manuscript preparation; All authors discussed the results, analyzed data and commented on the manuscript; QP, YC, and XM developed the concepts and designed the study. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, nos. 82170407, 81870580); the Guangdong Basic and Applied Basic Research Foundation (2021A1515010982, 2020A1515010089, 2022A1515012414); the Finance fund for science and technology special competitive allocation project of Zhanjiang city (no. 2021A05244); the Guangdong Key Laboratory of Age-related Cardiac and Cerebral Diseases (Exosome Transformation Laboratory of Neurological Diseases) and Exosome Research Platform (no. CLP2021A003); the “Clinical Medicine” + Science and Technology Cooperation Project of the Affiliated Hospital of Guangdong Medical University (no. CLP2021B005); and the PhD initiation Project of the Affiliated Hospital of Guangdong Medical University (no. 2021023563).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
All animal studies were approved by the Laboratory Animal Care and Use Committees at Guangdong Medical University. All procedures were performed in accordance with Guangdong Medical University’s guidelines.
Consent to Participate
All authors approved to participate.
Consent for Publication
All authors approved the publication.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary materials 1:
Fig.S1 Characterization of EPC-EXs. (A) Immunofluorescence images of GFP marker expression in EPCs after lentivirus infection. Scale bar, 50 μm. (B) EXs specific marker CD63 and TSG101 were measured by western blotting. (C) The numbers and size of EPC-EXs were detected by NTA. (D) The morphology of EPC-EXs was detected by TEM. Scale bar, 200 μm. (ZIP 12634 kb). Fig. S2 EPC-EXs distribution and metabolism in cerebral infarct area after tail vein injection. (A) Images showing PKH26 labeled EPC-EXs (red) merged into cerebral neurons (NEUN, green) and astrocytes (GFAP, green). Scale bar, 30 μm. (B) The metabolism of EPC-EXs (PKH26, red) in cerebral ECs (CD31, green) and infarct area were detected by immunofluorescence staining. Scale bar, 30 μm.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, Q., Wang, Y., Liu, J. et al. MiR-17-5p Mediates the Effects of ACE2-Enriched Endothelial Progenitor Cell-Derived Exosomes on Ameliorating Cerebral Ischemic Injury in Aged Mice. Mol Neurobiol 60, 3534–3552 (2023). https://doi.org/10.1007/s12035-023-03280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03280-4