Abstract
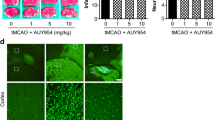
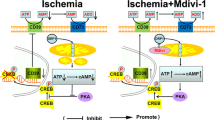
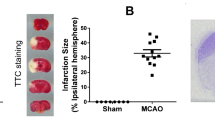
Phosphodiesterase 10A (PDE10A) hydrolyzes adenosine 3′,5′-cyclic monophosphate (cAMP) and guanosine 3′,5′-cyclic monophosphate (cGMP). It is highly expressed in the striatum. Recent evidence implied that PDE10A may be involved in the inflammatory processes following injury, such as ischemic stroke. Its role in ischemic injury was unknown. Herein, we exposed mice to 90 or 30-min middle cerebral artery occlusion, followed by the delivery of the highly selective PDE10A inhibitor TAK-063 (0.3 mg/kg or 3 mg/kg) immediately after reperfusion. Animals were sacrificed after 24 or 72 h, respectively. Both TAK-063 doses enhanced neurological function, reduced infarct volume, increased neuronal survival, reduced brain edema, and increased blood–brain barrier integrity, alongside cerebral microcirculation improvements. Post-ischemic neuroprotection was associated with increased phosphorylation (i.e., activation) of pro-survival Akt, Erk-1/2, GSK-3α/β and anti-apoptotic Bcl-xL abundance, decreased phosphorylation of pro-survival mTOR, and HIF-1α, MMP-9 and pro-apoptotic Bax abundance. Interestingly, PDE10A inhibition reduced inflammatory cytokines/chemokines, including IFN-γ and TNF-α, analyzed by planar surface immunoassay. In addition, liquid chromatography-tandem mass spectrometry revealed 40 proteins were significantly altered by TAK-063. Our study established PDE10A as a target for ischemic stroke therapy.






Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, Omori K (1999) Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J Biol Chem 274(26):18438–18445. https://doi.org/10.1074/jbc.274.26.18438
Soderling SH, Bayuga SJ, Beavo JA (1999) Isolation and characterization of a dual-substrate phosphodiesterase gene family: PDE10A. Proc Natl Acad Sci U S A 96(12):7071–7076. https://doi.org/10.1073/pnas.96.12.7071
Kelly MP (2018) Cyclic nucleotide signaling changes associated with normal aging and age-related diseases of the brain. Cell Signal 42:281–291. https://doi.org/10.1016/j.cellsig.2017.11.004
Cardinale A, Fusco FR (2018) Inhibition of phosphodiesterases as a strategy to achieve neuroprotection in Huntington’s disease. CNS Neurosci Ther 24(4):319–328. https://doi.org/10.1111/cns.12834
Persson J, Szalisznyo K, Antoni G, Wall A, Fallmar D, Zora H, Boden R (2020) Phosphodiesterase 10A levels are related to striatal function in schizophrenia: a combined positron emission tomography and functional magnetic resonance imaging study. Eur Arch Psychiatry Clin Neurosci 270(4):451–459. https://doi.org/10.1007/s00406-019-01021-0
Xie Z, Adamowicz WO, Eldred WD, Jakowski AB, Kleiman RJ, Morton DG, Stephenson DT, Strick CA, Williams RD, Menniti FS (2006) Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience 139(2):597–607. https://doi.org/10.1016/j.neuroscience.2005.12.042
Seeger TF, Bartlett B, Coskran TM, Culp JS, James LC, Krull DL, Lanfear J, Ryan AM, Schmidt CJ, Strick CA, Varghese AH, Williams RD, Wylie PG, Menniti FS (2003) Immunohistochemical localization of PDE10A in the rat brain. Brain Res 985(2):113–126. https://doi.org/10.1016/s0006-8993(03)02754-9
Hebb AL, Robertson HA, Denovan-Wright EM (2004) Striatal phosphodiesterase mRNA and protein levels are reduced in Huntington’s disease transgenic mice prior to the onset of motor symptoms. Neuroscience 123(4):967–981. https://doi.org/10.1016/j.neuroscience.2003.11.009
Giampa C, Laurenti D, Anzilotti S, Bernardi G, Menniti FS, Fusco FR (2010) Inhibition of the striatal specific phosphodiesterase PDE10A ameliorates striatal and cortical pathology in R6/2 mouse model of Huntington’s disease. PLoS ONE 5(10):e13417. https://doi.org/10.1371/journal.pone.0013417
Lee YY, Park JS, Leem YH, Park JE, Kim DY, Choi YH, Park EM, Kang JL, Kim HS (2019) The phosphodiesterase 10 inhibitor papaverine exerts anti-inflammatory and neuroprotective effects via the PKA signaling pathway in neuroinflammation and Parkinson’s disease mouse models. J Neuroinflammation 16(1):246. https://doi.org/10.1186/s12974-019-1649-3
Ito M, Aswendt M, Lee AG, Ishizaka S, Cao Z, Wang EH, Levy SL, Smerin DL, McNab JA, Zeineh M, Leuze C, Goubran M, Cheng MY, Steinberg GK (2018) RNA-sequencing analysis revealed a distinct motor cortex transcriptome in spontaneously recovered mice after stroke. Stroke 49(9):2191–2199. https://doi.org/10.1161/STROKEAHA.118.021508
Birjandi SZ, Abduljawad N, Nair S, Dehghani M, Suzuki K, Kimura H, Carmichael ST (2021) Phosphodiesterase 10A inhibition leads to brain region-specific recovery based on stroke type. Transl Stroke Res 12(2):303–315. https://doi.org/10.1007/s12975-020-00819-8
Suzuki K, Harada A, Suzuki H, Miyamoto M, Kimura H (2016) TAK-063, a PDE10A inhibitor with balanced activation of direct and indirect pathways, provides potent antipsychotic-like effects in multiple paradigms. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 41(9):2252–2262. https://doi.org/10.1038/npp.2016.20
Goldsmith P, Affinito J, McCue M, Tsai M, Roepcke S, Xie J, Gertsik L, Macek TA (2017) A Randomized multiple dose pharmacokinetic study of a novel PDE10A inhibitor TAK-063 in subjects with stable schizophrenia and Japanese subjects and modeling of exposure relationships to adverse events. Drugs R D 17(4):631–643. https://doi.org/10.1007/s40268-017-0214-8
Yurgelun-Todd DA, Renshaw PF, Goldsmith P, Uz T, Macek TA (2019) A randomized, placebo-controlled, phase 1 study to evaluate the effects of TAK-063 on ketamine-induced changes in fMRI BOLD signal in healthy subjects. Psychopharmacology. https://doi.org/10.1007/s00213-019-05366-1
Harada A, Suzuki K, Kamiguchi N, Miyamoto M, Tohyama K, Nakashima K, Taniguchi T, Kimura H (2015) Characterization of binding and inhibitory properties of TAK-063, a novel phosphodiesterase 10A inhibitor. PLoS ONE 10(3):e0122197. https://doi.org/10.1371/journal.pone.0122197
Suzuki K, Kimura H (2018) TAK-063, a novel PDE10A inhibitor with balanced activation of direct and indirect pathways, provides a unique opportunity for the treatment of schizophrenia. CNS Neurosci Ther 24(7):604–614. https://doi.org/10.1111/cns.12798
Kunitomo J, Yoshikawa M, Fushimi M, Kawada A, Quinn JF, Oki H, Kokubo H, Kondo M, Nakashima K, Kamiguchi N, Suzuki K, Kimura H, Taniguchi T (2014) Discovery of 1-[2-fluoro-4-(1H-pyrazol-1-yl)phenyl]-5-methoxy-3-(1-phenyl-1H-pyrazol-5-yl)pyri dazin-4(1H)-one (TAK-063), a highly potent, selective, and orally active phosphodiesterase 10A (PDE10A) inhibitor. J Med Chem 57(22):9627–9643. https://doi.org/10.1021/jm5013648
Beker MC, Caglayan AB, Kelestemur T, Caglayan B, Yalcin E, Yulug B, Kilic U, Hermann DM, Kilic E (2015) Effects of normobaric oxygen and melatonin on reperfusion injury: role of cerebral microcirculation. Oncotarget 6(31):30604–30614. https://doi.org/10.18632/oncotarget.5773
Caglayan AB, Beker MC, Caglayan B, Yalcin E, Caglayan A, Yulug B, Hanoglu L, Kutlu S, Doeppner TR, Hermann DM, Kilic E (2019) Acute and post-acute neuromodulation induces stroke recovery by promoting survival signaling, neurogenesis, and pyramidal tract plasticity. Front Cell Neurosci 13:144. https://doi.org/10.3389/fncel.2019.00144
Beker MC, Caglayan B, Caglayan AB, Kelestemur T, Yalcin E, Caglayan A, Kilic U, Baykal AT, Reiter RJ, Kilic E (2019) Interaction of melatonin and Bmal1 in the regulation of PI3K/AKT pathway components and cellular survival. Sci Rep 9(1):19082. https://doi.org/10.1038/s41598-019-55663-0
Wisniewski JR, Zougman A, Nagaraj N, Mann M (2009) Universal sample preparation method for proteome analysis. Nat Methods 6(5):359–362. https://doi.org/10.1038/nmeth.1322
Yalcin E, Beker MC, Turkseven S, Caglayan B, Gurel B, Kilic U, Caglayan AB, Kalkan R, Baykal AT, Kelestemur T, Kilic E (2019) Evidence that melatonin downregulates Nedd4-1 E3 ligase and its role in cellular survival. Toxicol Appl Pharmacol 379:114686. https://doi.org/10.1016/j.taap.2019.114686
Beker MC, Caglayan B, Yalcin E, Caglayan AB, Turkseven S, Gurel B, Kelestemur T, Sertel E, Sahin Z, Kutlu S, Kilic U, Baykal AT, Kilic E (2018) Time-of-Day dependent neuronal injury after ischemic stroke: implication of circadian clock transcriptional factor Bmal1 and survival kinase AKT. Mol Neurobiol 55(3):2565–2576. https://doi.org/10.1007/s12035-017-0524-4
Acioglu C, Mirabelli E, Baykal AT, Ni L, Ratnayake A, Heary RF, Elkabes S (2016) Toll like receptor 9 antagonism modulates spinal cord neuronal function and survival: direct versus astrocyte-mediated mechanisms. Brain Behav Immun 56:310–324. https://doi.org/10.1016/j.bbi.2016.03.027
Kilic E, Bahr M, Hermann DM (2001) Effects of recombinant tissue plasminogen activator after intraluminal thread occlusion in mice: role of hemodynamic alterations. Stroke 32(11):2641–2647. https://doi.org/10.1161/hs1101.097381
Bacigaluppi M, Pluchino S, Peruzzotti-Jametti L, Kilic E, Kilic U, Salani G, Brambilla E, West MJ, Comi G, Martino G, Hermann DM (2009) Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 132(Pt 8):2239–2251. https://doi.org/10.1093/brain/awp174
Huan X, Oumei C, Hongmei Q, Junxia Y, Xiaojiao M, Qingsong J (2019) PDE9 inhibition promotes proliferation of neural stem cells via cGMP-PKG pathway following oxygen-glucose deprivation/reoxygenation injury in vitro. Neurochem Int 133:104630. https://doi.org/10.1016/j.neuint.2019.104630
Omori K, Kotera J (2007) Overview of PDEs and their regulation. Circ Res 100(3):309–327. https://doi.org/10.1161/01.RES.0000256354.95791.f1
Russwurm C, Koesling D, Russwurm M (2015) Phosphodiesterase 10A is tethered to a synaptic signaling complex in striatum. J Biol Chem 290(19):11936–11947. https://doi.org/10.1074/jbc.M114.595769
Tejeda GS, Whiteley EL, Deeb TZ, Burli RW, Moss SJ, Sheridan E, Brandon NJ, Baillie GS (2020) Chorea-related mutations in PDE10A result in aberrant compartmentalization and functionality of the enzyme. Proc Natl Acad Sci USA 117(1):677–688. https://doi.org/10.1073/pnas.1916398117
Knopp C, Hausler M, Muller B, Damen R, Stoppe A, Mull M, Elbracht M, Kurth I, Begemann M (2019) PDE10A mutation in two sisters with a hyperkinetic movement disorder—response to levodopa. Parkinsonism Relat Disord 63:240–242. https://doi.org/10.1016/j.parkreldis.2019.02.007
VerPlank JJS, Tyrkalska SD, Fleming A, Rubinsztein DC, Goldberg AL (2020) cGMP via PKG activates 26S proteasomes and enhances degradation of proteins, including ones that cause neurodegenerative diseases. Proc Natl Acad Sci USA 117(25):14220–14230. https://doi.org/10.1073/pnas.2003277117
Zhang H, Pan B, Wu P, Parajuli N, Rekhter MD, Goldberg AL, Wang X (2019) PDE1 inhibition facilitates proteasomal degradation of misfolded proteins and protects against cardiac proteinopathy. Science advances 5(5):eaaw5870. https://doi.org/10.1126/sciadv.aaw5870
Myeku N, Duff KE (2018) Targeting the 26S proteasome to protect against proteotoxic diseases. Trends Mol Med 24(1):18–29. https://doi.org/10.1016/j.molmed.2017.11.006
Krueger M, Mages B, Hobusch C, Michalski D (2019) Endothelial edema precedes blood-brain barrier breakdown in early time points after experimental focal cerebral ischemia. Acta Neuropathol Commun 7(1):17. https://doi.org/10.1186/s40478-019-0671-0
Uzdensky AB (2020) Regulation of apoptosis in the ischemic penumbra in the first day post-stroke. Neural Regen Res 15(2):253–254. https://doi.org/10.4103/1673-5374.265546
Xing C, Arai K, Lo EH, Hommel M (2012) Pathophysiologic cascades in ischemic stroke. Int J Stroke 7(5):378–385. https://doi.org/10.1111/j.1747-4949.2012.00839.x
Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W (1989) Continuous measurement of cerebral cortical blood flow by laser-Doppler flowmetry in a rat stroke model. J. Cereb 9(5):589–596. https://doi.org/10.1038/jcbfm.1989.84
Hedna VS, Ansari S, Shahjouei S, Cai PY, Ahmad AS, Mocco J, Qureshi AI (2015) Validity of laser Doppler flowmetry in predicting outcome in murine intraluminal middle cerebral artery occlusion stroke. J Vasc Interv Neurol 8(3):74–82
Shi X, Wang J, Lei Y, Cong C, Tan D, Zhou X (2019) Research progress on the PI3K/AKT signaling pathway in gynecological cancer (Review). Mol Med Rep 19(6):4529–4535. https://doi.org/10.3892/mmr.2019.10121
Carracedo A, Pandolfi PP (2008) The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene 27(41):5527–5541. https://doi.org/10.1038/onc.2008.247
Kilic U, Caglayan AB, Beker MC, Gunal MY, Caglayan B, Yalcin E, Kelestemur T, Gundogdu RZ, Yulug B, Yilmaz B, Kerman BE, Kilic E (2017) Particular phosphorylation of PI3K/Akt on Thr308 via PDK-1 and PTEN mediates melatonin’s neuroprotective activity after focal cerebral ischemia in mice. Redox Biol 12:657–665. https://doi.org/10.1016/j.redox.2017.04.006
Gao X, Zhang H, Steinberg G, Zhao H (2010) The Akt pathway is involved in rapid ischemic tolerance in focal ischemia in rats. Transl Stroke Res 1(3):202–209. https://doi.org/10.1007/s12975-010-0017-5
LiCausi F, Hartman NW (2018) Role of mTOR complexes in neurogenesis. Int J Mol Sci 19(5):1544. https://doi.org/10.3390/ijms19051544
Amalia L, Sadeli HA, Parwati I, Rizal A, Panigoro R (2020) Hypoxia-inducible factor-1alpha in acute ischemic stroke: neuroprotection for better clinical outcome. Heliyon 6(6):e04286. https://doi.org/10.1016/j.heliyon.2020.e04286
Davis CK, Jain SA, Bae ON, Majid A, Rajanikant GK (2018) Hypoxia mimetic agents for ischemic stroke. Dev. Biol 6:175. https://doi.org/10.3389/fcell.2018.00175
Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC (2007) Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J. Neurosci 27(23):6320–6332. https://doi.org/10.1523/JNEUROSCI.0449-07.2007
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y (2018) Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 163–164:144–171. https://doi.org/10.1016/j.pneurobio.2017.10.001
Turner RJ, Sharp FR (2016) Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front Cell Neurosci 10:56. https://doi.org/10.3389/fncel.2016.00056
D’Orsi B, Mateyka J, Prehn JHM (2017) Control of mitochondrial physiology and cell death by the Bcl-2 family proteins Bax and Bok. Neurochem Int 109:162–170. https://doi.org/10.1016/j.neuint.2017.03.010
Doll DN, Barr TL, Simpkins JW (2014) Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis 5(5):294–306. https://doi.org/10.14336/AD.2014.0500294
Li KW, Ganz AB, Smit AB (2019) Proteomics of neurodegenerative diseases: analysis of human post-mortem brain. J Neurochem 151(4):435–445. https://doi.org/10.1111/jnc.14603
Zhang X, Wang X, Khurm M, Zhan G, Zhang H, Ito Y, Guo Z (2020) Alterations of brain quantitative proteomics profiling revealed the molecular mechanisms of diosgenin against cerebral ischemia reperfusion effects. J Proteome Res 19(3):1154–1168. https://doi.org/10.1021/acs.jproteome.9b00667
Rodrigues-Amorim D, Rivera-Baltanas T, Vallejo-Curto MDC, Rodriguez-Jamardo C, de Las HE, Barreiro-Villar C, Blanco-Formoso M, Fernandez-Palleiro P, Alvarez-Ariza M, Lopez M, Garcia-Caballero A, Olivares JM, Spuch C (2019) Proteomics in schizophrenia: a gateway to discover potential biomarkers of psychoneuroimmune pathways. Front Psych 10:885. https://doi.org/10.3389/fpsyt.2019.00885
Funding
This work was supported by TUBITAK (The Scientific and Technological Research Council of Turkey/ 218S453; MCB) and Turkish Academy of Sciences (TUBA; EK).
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all authors. MCB, ABC, and SA carried out experimental work, analyzed data, and helped to write the manuscript. EO and TK performed LC–MS/MS experiments and analyzed data. NA and BC carried out Western blot and immunofluorescence studies. MCB, TRD, UK, DMH, and EK defined the research theme and revised the manuscript critically.
Corresponding author
Ethics declarations
Ethics Approval
Experiments were performed in accordance to National Institutes of Health (NIH) guidelines for the care and use of laboratory animals and approved by local government authorities (Istanbul Medipol University, Animal Research Ethics Committee).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beker, M.C., Caglayan, A.B., Altunay, S. et al. Phosphodiesterase 10A Is a Critical Target for Neuroprotection in a Mouse Model of Ischemic Stroke. Mol Neurobiol 59, 574–589 (2022). https://doi.org/10.1007/s12035-021-02621-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02621-5