Abstract
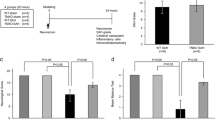
Tenascin-C (TNC), a matricellular protein, is upregulated in brain parenchyma after experimental subarachnoid hemorrhage (SAH). Recent studies emphasize that early brain injury (EBI) should be overcome to improve post-SAH outcomes. The aim of this study was to investigate effects of TNC knockout (TNKO) on neuronal apoptosis and neuroinflammation, both of which are important constituents of EBI after SAH. C57BL/6 wild-type (WT) mice or TNKO mice underwent sham or filament perforation SAH modeling. Twenty-five WT mice and 25 TNKO mice were randomly divided into sham+WT (n = 10), sham+TNKO (n = 8), SAH+WT (n = 15), and SAH+TNKO (n = 17) groups. Beam balance test, neurological score, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining, immunostaining of Toll-like receptor 4 (TLR4), and Western blotting were performed to evaluate neurobehavioral impairments, neuronal apoptosis, and neuroinflammation at 24 h post-SAH. Deficiency of TNC significantly alleviated post-SAH neurobehavioral impairments and neuronal apoptosis. The protective effects of TNKO on neurons were associated with the inhibition of a caspase-dependent apoptotic pathway, which was at least partly mediated by TLR4/nuclear factor-κB/interleukin-1β and interleukin-6 signaling cascades. This study first provided the direct evidence that TNC causes post-SAH neuronal apoptosis and neuroinflammation, potentially leading to the development of a new molecular targeted therapy against EBI.





Similar content being viewed by others
References
Zacharia BE, Hickman ZL, Grobelny BT, DeRosa P, Kotchetkov I, Ducruet AF, Connolly ES Jr (2010) Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 21(2):221–233. https://doi.org/10.1016/j.nec.2009.10.002
Chen S, Feng H, Sherchan P, Klebe D, Zhao G, Sun X, Zhang J, Tang J et al (2014) Controversies and evolving new mechanisms in subarachnoid hemorrhage. Prog Neurobiol 115:64–91. https://doi.org/10.1016/j.pneurobio.2013.09.002
Sehba FA, Hou J, Pluta RM, Zhang JH (2012) The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol 97(1):14–37. https://doi.org/10.1016/j.pneurobio.2012.02.003
Midwood KS, Chiquet M, Tucker RP, Orend G (2016) Tenascin-C at a glance. J Cell Sci 129(23):4321–4327. https://doi.org/10.1242/jcs.190546
Udalova IA, Ruhmann M, Thomson SJ, Midwood KS (2011) Expression and immune function of tenascin-C. Crit Rev Immunol 31(2):115–145
Liu L, Kawakita F, Fujimoto M, Nakano F, Imanaka-Yoshida K, Yoshida T, Suzuki H (2017) Role of periostin in early brain injury after subarachnoid hemorrhage in mice. Stroke 48(4):1108–1111. https://doi.org/10.1161/STROKEAHA.117.016629
Suzuki H, Kinoshita N, Imanaka-Yoshida K, Yoshida T, Taki W (2008) Cerebrospinal fluid tenascin-C increases preceding the development of chronic shunt-dependent hydrocephalus after subarachnoid hemorrhage. Stroke 39(5):1610–1612. https://doi.org/10.1161/STROKEAHA.107.505735
Suzuki H, Kanamaru K, Shiba M, Fujimoto M, Imanaka-Yoshida K, Yoshida T, Taki W (2011) Cerebrospinal fluid tenascin-C in cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg Anesthesiol 23(4):310–317. https://doi.org/10.1097/ANA.0b013e31822aa1f2
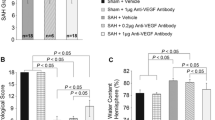
Liu L, Fujimoto M, Kawakita F, Nakano F, Imanaka-Yoshida K, Yoshida T, Suzuki H (2016) Anti-vascular endothelial growth factor treatment suppresses early brain injury after subarachnoid hemorrhage in mice. Mol Neurobiol 53(7):4529–4538. https://doi.org/10.1007/s12035-015-9386-9
Shiba M, Suzuki H, Fujimoto M, Shimojo N, Imanaka-Yoshida K, Yoshida T, Kanamaru K, Matsushima S et al (2012) Imatinib mesylate prevents cerebral vasospasm after subarachnoid hemorrhage via inhibiting tenascin-C expression in rats. Neurobiol Dis 46(1):172–179. https://doi.org/10.1016/j.nbd.2012.01.005
Fujimoto M, Shiba M, Kawakita F, Liu L, Shimojo N, Imanaka-Yoshida K, Yoshida T, Suzuki H (2016) Deficiency of tenascin-C and attenuation of blood-brain barrier disruption following experimental subarachnoid hemorrhage in mice. J Neurosurg 124(6):1693–1702. https://doi.org/10.3171/2015.4.JNS15484
Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S (1992) Mice develop normally without tenascin. Genes Dev 6(10):1821–1831
Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH (2010) Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann Neurol 68(5):650–660. https://doi.org/10.1002/ana.22102
Suzuki H, Zhang JH (2012) Neurobehavioral assessments of subarachnoid hemorrhage. In: Chen J, Xu X-M, Xu ZC, Zhang JH (eds) Springer protocols handbooks. Animal models of acute neurological injuries II. Humana Press, New York, pp. 435–440. https://doi.org/10.1007/978-1-61779-576-3_31
Sugawara T, Ayer R, Jadhav V, Zhang JH (2008) A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 167(2):327–334. https://doi.org/10.1016/j.jneumeth.2007.08.004
Shiba M, Fujimoto M, Imanaka-Yoshida K, Yoshida T, Taki W, Suzuki H (2014) Tenascin-C causes neuronal apoptosis after subarachnoid hemorrhage in rats. Transl Stroke Res 5(2):238–247. https://doi.org/10.1007/s12975-014-0333-2
Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH (2004) Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 24(8):916–925. https://doi.org/10.1097/01.WCB.0000125886.48838.7E
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26(11):1341–1353. https://doi.org/10.1038/sj.jcbfm.9600283
Suzuki H, Kawakita F (2016) Tenascin-C in aneurysmal subarachnoid hemorrhage: Deleterious or protective? Neural Regen Res 11(2):230–231. https://doi.org/10.4103/1673-5374.177721
Fujimoto M, Shiba M, Kawakita F, Liu L, Shimojo N, Imanaka-Yoshida K, Yoshida T, Suzuki H (2017) Effects of tenascin-C knockout on cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol 55:1951–1958. https://doi.org/10.1007/s12035-017-0466-x
Okada T, Suzuki H (2017) Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal subarachnoid hemorrhage. Neural Regen Res 12(2):193–196. https://doi.org/10.4103/1673-5374.200795
Hanafy KA (2013) The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation 10:83. https://doi.org/10.1186/1742-2094-10-83
Kawakita F, Fujimoto M, Liu L, Nakano F, Nakatsuka Y, Suzuki H (2017) Effects of Toll-like receptor 4 antagonists against cerebral vasospasm after experimental subarachnoid hemorrhage in mice. Mol Neurobiol 54(8):6624–6633. https://doi.org/10.1007/s12035-016-0178-7
Zhou CH, Wang CX, Xie GB, Wu LY, Wei YX, Wang Q, Zhang HS, Hang CH et al (2015) Fisetin alleviates early brain injury following experimental subarachnoid hemorrhage in rats possibly by suppressing TLR4/NF-κB signaling pathway. Brain Res 1629:250–259. https://doi.org/10.1016/j.brainres.2015.10.016
Xia DY, Zhang HS, Wu LY, Zhang XS, Zhou ML, Hang CH (2017) Pentoxifylline alleviates early brain injury after experimental subarachnoid hemorrhage in rats: possibly via inhibiting TLR4/NF-κB signaling pathway. Neurochem Res 42(4):963–974. https://doi.org/10.1007/s11064-016-2129-0
Roy A, Srivastava M, Saqib U, Liu D, Faisal SM, Sugathan S, Bishnoi S, Baig MS (2016) Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int Immunopharmacol 40:79–89. https://doi.org/10.1016/j.intimp.2016.08.026
Guadagno J, Swan P, Shaikh R, Cregan SP (2015) Microglia-derived IL-1β triggers p53-mediated cell cycle arrest and apoptosis in neural precursor cells. Cell Death Dis 6:e1779. https://doi.org/10.1038/cddis.2015.151
Tan Y, Uchida K, Nakajima H, Guerrero AR, Watanabe S, Hirai T, Takeura N, Liu SY et al (2013) Blockade of interleukin 6 signaling improves the survival rate of transplanted bone marrow stromal cells and increases locomotor function in mice with spinal cord injury. J Neuropathol Exp Neurol 72(10):980–993. https://doi.org/10.1097/NEN.0b013e3182a79de9
Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N et al (2009) Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 15(7):774–780. https://doi.org/10.1038/nm.1987
Fujimoto M, Suzuki H, Shiba M, Shimojo N, Imanaka-Yoshida K, Yoshida T, Kanamaru K, Matsushima S et al (2013) Tenascin-C induces prolonged constriction of cerebral arteries in rats. Neurobiol Dis 55:104–109. https://doi.org/10.1016/j.nbd.2013.01.007
Fujimoto M, Shiba M, Kawakita F, Liu L, Nakasaki A, Shimojo N, Imanaka-Yoshida K, Yoshida T et al (2016) Epidermal growth factor-like repeats of tenascin-C-induced constriction of cerebral arteries via activation of epidermal growth factor receptors in rats. Brain Res 1642:436–444. https://doi.org/10.1016/j.brainres.2016.04.034
Van Obberghen-Schilling E, Tucker RP, Saupe F, Gasser I, Cseh B, Orend G (2011) Fibronectin and tenascin-C: accomplices in vascular morphogensis during development and tumor growth. Int J Dev Biol 55:511–525. https://doi.org/10.1387/ijdb.103243eo
Acknowledgements
We thank Ms. Chiduru Yamamoto (Department of Neurosurgery, Mie University Graduate School of Medicine) for her technical assistance.
Funding
This study was funded by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science to Dr. Fujimoto (15 K19962), and Mie Medical Research Foundation to Dr. Suzuki.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Animals
All procedures were approved by the Animal Ethics Review Committee of Mie University and were carried out according to the institution’s Guidelines for Animal Experiments.
Electronic supplementary material
ESM 1
(PDF 961 kb)
Rights and permissions
About this article
Cite this article
Liu, L., Fujimoto, M., Nakano, F. et al. Deficiency of Tenascin-C Alleviates Neuronal Apoptosis and Neuroinflammation After Experimental Subarachnoid Hemorrhage in Mice. Mol Neurobiol 55, 8346–8354 (2018). https://doi.org/10.1007/s12035-018-1006-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1006-z