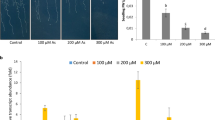
Abstract
Aluminum (Al) stress represses mitochondrial respiration and produces reactive oxygen species (ROS) in plants. Mitochondrial alternative oxidase (AOX) uncouples respiration from mitochondrial ATP production and may improve plant performance under Al stress by preventing excess accumulation of ROS. We tested respiratory changes and ROS production in isolated mitochondria and whole cell of tobacco (SL, ALT 301) under Al stress. Higher capacities of AOX pathways relative to cytochrome pathways were observed in both isolated mitochondria and whole cells of ALT301 under Al stress. AOX1 when studied showed higher AOX1 expression in ALT 301 than SL cells under stress. In order to study the function of tobacco AOX gene under Al stress, we produced transformed tobacco cell lines by introducing NtAOX1 expressed under the control of the cauliflower mosaic virus (CaMV) 35 S promoter in sensitive (SL) Nicotiana tabacum L. cell lines. The enhancement of endogenous AOX1 expression and AOX protein with or without Al stress was in the order of transformed tobacco cell lines > ALT301 > wild type (SL). A decreased respiratory inhibition and reduced ROS production with a better growth capability were the significant features that characterized AOX1 transformed cell lines under Al stress. These results demonstrated that AOX plays a critical role in Al stress tolerance with an enhanced respiratory capacity, reducing mitochondrial oxidative stress burden and improving the growth capability in tobacco cells.









Similar content being viewed by others
References
Affourtit, C., Albury, M. S., Crichton, P. G., & Moore, A. L. (2002). Exploring the molecular nature of alternative oxidase regulation and catalysis. FEBS Letters, 510, 121–126.
Amirsadeghi, S., Robson, C. A., McDonald, A. E., & Vanlerberghe, G. C. (2006). Changes in plant mitochondrial electron transport alter cellular levels of reactive oxygen species and susceptibility to cell death signaling molecules. Plant Cell Physiology, 47, 1509–1519.
Amirsadeghi, S., Robson, C. A., & Vanlerberghe, G. C. (2007). The role of the mitochondrion in plant responses to biotic stress. Physiologia Plantarum, 129, 253–266.
An, G., Evert, P. R., Mitra, A., & Ha, S. B. (1988). Plant molecular biology manual. Dordrecht: Kluwer.
Bartoli, C. G., Gomez, F., Gergoff, G., Guiamét, J. J., & Puntarulo, S. (2005). Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. Journal of Experimental Botany, 56(415), 1269–1276.
Brown, R. C., & Lemmon, B. E. (1995). Methods in plant immunolight microscopy. Methods in Cell Biology, 49, 85–107.
Chiu, W. L., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., & Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Current Biology, 6, 325–330.
Escobar, M. A., Geisler, D. A., & Rasmusson, A. G. (2006). Reorganization of the alternative pathways of the arabidopsis respiratory chain by nitrogen supply: Opposing effects of ammonium and nitrate. Plant J, 45, 775–788.
Finnegan, P. F., Soole, K. L., & Umbach, A. L. (2004). Alternative mitochondrial electron transport proteins in higher plants. In Day, D. A., Millar, H., Whelan, J. (Eds.), Plant mitochondria: From genome to function (Vol. 17, pp. 163–230). Dordrecht: Kluwer Academic.
Fiorani, F., Umbach, A. L., & Siedow, J. N. (2005). The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1 a transgenic plants. Plant Physiology, 139, 1795–1805.
Gao, C., Xing, D., Li, L., & Zhang, L. (2008). Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta, 227, 755–767.
Gardner, P. R., Raineri, I., Epstein, L. B., & White, C. W. (1995). Superoxide and iron modulate aconitase activity in mammalian cells. The Journal or Biological Chemistry, 270, 13399–13405.
Gonzàlez-Meler, M. A., Ribas-Carbo, M., Giles, L., & Siedow, J. N. (1999). The effect of growth and measurement temperature on the activity of the alternative respiratory pathway. Plant Physiology, 120, 765–772.
Guy, R. D., & Vanlerberghe, G. C. (2005). Partitioning of respiratory electrons in the ark in leaves of transgenic tobacco with modified levels of alternative oxidase. Physiologia Plantarum, 125(2), 171–180.
Hilal, M., Castagnaro, A., Moreno, H., & Massa, E. M. (1997). Specific localization of the respiratory alternative oxidase in meristematic and xylematic tissues from developing soybean roots and hypocotyls. Plant Physiology, 115, 1499–1503.
Ikegawa, H., Yamamoto, Y., & Matsumoto, H. (2000). Responses to aluminium of suspension-cultured tobacco cells in simple calcium solution. Soil Science Plant Nutrition, 46, 503–514.
Karpova, O. V., Kuzmin, E. V., Elthon, T. E., & Newton, K. J. (2002). Differential expression of alternative oxidase genes in maize mitochondrial mutants. Plant Cell, 14, 3271–3284.
Kawano, T., Kadono, T., Furuichi, T., Muto, S., & Lapeyrie, F. (2003). Aluminium induced distortion in calcium signaling involving oxidative bursts and channel regulation in tobacco BY-2 cells. Biochemical and Biophysical Research Communications, 308, 35–42.
Kobayashi, F., Takumi, S., Nakata, M., Ohno, R., Nakamura, T., & Nakamura, C. (2004). Comparative study of the expression profiles of the Cor/Lea gene family in two wheat cultivars with contrasting levels of freezing tolerance. Physiologia Plantarum, 120, 585–594.
Kobayashi, Y., Hoekenga, O. A., Itoh, H., Nakashima, M., Saito, S., Shaff, J. E., et al. (2007). Characterization of AtALMT1 expression in aluminum-inducible malate release and its role for rhizotoxic stress tolerance in arabidopsis. Plant Physiology, 145, 843–852.
Lambers, H. (1982). Cyanide-resistant respiration: A non-phosphorylating electron transport pathway acting as an energy overflow. Plant Physiology, 55, 478–485.
Li, Z., & Xing, D. (2011). Mechanistic study of mitochondria-dependent programmed cell death induced by aluminium phytotoxicity using fluorescence techniques. Journal of Experimental Botany, 62(1), 331–343.
Li, Z., & Xing, D. (2010). Mitochondrial pathway leading to programmed cell death induced by aluminum phytotoxicity in arabidopsis. Plant Signaling & Behavior, 5(12), 660–662.
Maxwell, D. P., Nickels, R., & McIntosh, L. (2002). Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant Journal, 29, 269–279.
Maxwell, D. P., Wang, Y., & Mcintosh, L. (1999). The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proceedings of the National Academy of Sciences USA, 96, 8271–8276.
McCabe, T. C., Finnegan, P. M., Harvey Millar, A., Day, D. A., & Whelan, J. (1998). Differential expression of alternative oxidase genes in soybeancotyledons during postgerminative development. Plant Physiology, 118, 675–682.
McDonald, A. E. (2008). Alternative oxidase: An inter-kingdom perspective on the function and regulation of this broadly distributed ‘cyanide-resistant’ terminal oxidase. Functional Plant Biology, 35, 535–552.
Melis, M., Spangfort, M., & Andersson, B. (1987). Light-absorption and electron transport balance between photosystem II and photosystem I in spinach chloroplasts. Photochemistry and Photobiology, 45, 129–136.
Millar, A. H., Sweetlove, L. J., Giege, P., & Leaver, C. J. (2001). Analysis of the arabidopsis mitochondrial proteome. Plant Physiology, 127, 1711–1727.
Millar, A. H., Wiskich, J. T., Whelan, J., & Day, D. A. (1993). Organic acid activation of the alternative oxidase of plant mitochondria. FEBS Letters, 329, 259–262.
Millenaar, F. F., Roelofs, R., Gonzàlez-Meler, M. A., Siedow, J. N., Wagner, A. M., & Lambers, H. (2000). The alternative oxidase in roots of Poa annua after transfer from high-light to low-light conditions. Plant Journal, 23, 623–632.
Miller, G., Suzuki, N., Rizhsky, L., Hegie, A., Koussevitzky, S., & Mittler, R. (2007). Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiology, 144, 1777–1785.
Mittler, R., Vanderauwera, S., Gollery, M., & Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends in Plant Science, 9, 490–498.
Moore, A. L., Albury, M. S., Crichton, P. G., & Affourtit, C. (2002). Function of the alternative oxidase: Is it still a scavenger? Trends in Plant Science, 7, 478–481.
Neuberger, M. (1985). Preparation of plant mitochondria, criteria for assessment of mitochondrial integrity and purity, survival in vitro. In R. Douce & D. Day (Eds.), Higher plant cell respiration (pp. 7–24). Berlin: Springer.
Noctor, G., De Paepe, R., & Foyer, C. H. (2007). Mitochondrial redox biology and homeostasis n plants. Trends in Plant Science, 12, 125–134.
Onda, Y., Kato, Y., Abe, Y., Ito, T., Morohashi, M., Ito, Y., et al. (2008). Functional coexpression of the mitochondrial alternative oxidase and uncoupling protein underlies thermoregulation in the thermogenic florets of skunk cabbage. Plant Physiology, 146, 636–645.
Pan, J., Zhu, M., & Chen, H. (2001). Aluminium-induced cell death in root tip cells of barley. Environmental and Experimental Botany, 46, 71–79.
Panda, S. K., Baluska, F., & Matsumoto, H. (2009). Al stress signaling in plants. Plant Signaling & behavior, 4, 592–597.
Panda, S. K., & Matsumoto, H. (2010). Changes in antioxidant gene expression and induction of oxidative stress in pea (Pisum sativum L.) under Al stress. BioMetals, 23(4), 753–762.
Panda, S. K., & Matsumoto, H. (2007). Molecular physiology of aluminium toxicity and tolerance in plants. Botanical Review, 73(4), 326–347.
Panda, S. K., Singha, L. B., & Khan, M. H. (2003). Does aluminium phytotoxicity induce oxidative stress in greengram (Vigna radiata)? Bulgarian Journal of Plant Physiology, 29, 77–86.
Panda, S. K., Yamamoto, Y., Kondo, H., & Matsumoto, H. (2008). Mitochondrial alterations related to programmed cell death in tobacco cells under aluminium stress. Comptes Rendus Biologies, 331, 597–610.
Parsons, H. L., Yip, J. Y., & Vanlerberghe, G. C. (1999). Increased respiratory restriction during phosphate-limited growth in transgenic tobacco cells lacking alternative oxidase. Plant Physiology, 121, 1309–1320.
Piñeros, M. A., Cançado, G. M. A., & Kochian, L. V. (2008). Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: Functional and structural implications. Plant Physiology, 147, 2131–2146.
Purvis, A. C., & Shewfelt, R. L. (1993). Does the alternative pathway ameliorate chilling injury in sensitive tissues? Physiologia Plantarum, 88, 712–718.
Purvis, A. C. (1997). Role of alternative oxidase in limiting superoxide production by plant mitochondria. Physiologia Plantarum, 100, 165–170.
Rhoads, D. M., & Mcintosh, L. (1992). Salicylic acid regulation of respiration in higher plants: Alternative oxidase expression. Plant Cell, 4, 1131–1139.
Rhoads, D. M., Umbach, A. L., Subbaiah, C. C., & Siedow, J. N. (2006). Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiology, 141, 357–366.
Ribas-Carbo, M., Taylor, N. L., Giles, L., Busquets, S., Finnegan, P. M., Day, D. A., et al. (2005). Effects of water stress on respiration in soybean leaves. Plant Physiology, 139, 466–473.
Robson, C. A., & Vanlerberghe, G. C. (2002). Transgenic plant cells lacking mitochondrial alternative oxidase have increased susceptibility to mitochondria-dependent and-independent pathways of programmed cell death. Plant Physiology, 129, 1908–1920.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2 nd ed.). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Sasaki, T., Yamamoto, Y., Ezaki, B., Katsuhara, M., Ahn, S. J., Ryan, P. R., et al. (2004). A wheat gene encoding an aluminum-activated malate transporter. Plant Journal, 37, 645–653.
Scandalios, J. G. (1993). Oxygen stress and superoxide dismutase. Plant Physiology, 101, 7–12.
Simons, B. H., Millenaar, F. F., Mulder, L., Van Loon, L. C., & Lambers, H. (1999). Enhanced expression and activation of the alternative oxidase during infection of arabidopsis with Pseudomonas syringae pv tomato. Plant Physiology, 120, 529–538.
Snell, F. D., & Snell, C. T. (1949). Colorimetric methods of analysis. Inorganic, 2, 882–883.
Sugie, A., Naydenov, N., Mizuno, N., Nakamura, C., & Takumi, S. (2006). Overexpression of wheat alternative oxidase gene Waox1a alters respiration capacity and response to reactive oxygen species under low temperature in transgenic arabidopsis. Genes & Genetic Systems, 81, 349–354.
Taylor, N. L., Day, D. A., & Millar, A. H. (2002). Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. Journal of Biological Chemistry, 277, 42663–42668.
Umbach, A. L., Fiorani, F., & Siedow, J. N. (2005). Characterization of transformed arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiology, 139, 1806–1820.
Umbach, A. L., & Siedow, J. N. (1993). Covalent and noncovalent dimers of the cyanide-resistant alternative oxidase protein in higher plant mitochondria and their relationship to enzyme activity. Plant Physiology, 103, 845–854.
Vanlerberghe, G. C., & Mcintosh, L. (1997). Alternative oxidase: From gene to function. Annual Review of Plant Physiology and Plant Molecular Biology, 48, 703–734.
Vanlerberghe, G. C., & McIntosh, L. (1992). Lower growth temperature increases alternative pathway capacity and alternative oxidase protein in tobacco. Plant Physiology, 100, 115–119.
Vanlerberghe, G. C., & McIntosh, L. (1994). Mitochondrial electron transport regulation of nuclear gene expression: Studies with the alternative oxidase gene of tobacco. Plant Physiology, 105, 867–874.
Vanlerberghe, G. C., & McIntosh, L. (1996). Signals regulating the expression of the nuclear gene encoding alternative oxidase of plant mitochondria. Plant Physiology, 111, 589–595.
Vanlerberghe, G. C., Robson, C. A., & Yip, J. Y. H. (2002). Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulating the cytochrome pathway prevents programmed cell death. Plant Physiology, 129, 1829–1842.
Vanlerberghe, G. C., Vanlerberghe, A. E., & McIntosh, L. (1994). Molecular genetic alteration of plant respiration: Silencing and overexpression of alternative oxidase in transgenic tobacco. Plant Physiology, 106, 1503–1510.
Yamaguchi, Y., Yamamoto, Y., & Matsumoto, H. (1999). Cell death process initiated by a combination of aluminium and iron in suspension-cultured tobacco cells. Soil Science and Plant Nutrition, 45, 647–657.
Yamamoto, Y., Kobayashi, Y., Devi, S. R., Rikishi, S., & Matsumoto, H. (2002). Aluminium toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiology, 128, 63–72.
Yamamoto, Y., Rikishi, S., Chang, Y. C., Ono, K., Kasai, M., & Matsumoto, H. (1994). Quantitative estimation of aluminium toxicity in cultured tobacco cells: Correlation between aluminium uptake and growth inhibition. Plant and Cell Physiology, 35, 575–583.
Yoshida, K., Terashima, I., & Noguchi, K. (2006). Distinct roles of the cytochrome pathway and alternative oxidase in leaf photosynthesis. Plant and Cell Physiology, 47, 22–31.
Yoshida, K., Terashima, I., & Noguchi, K. (2007). Up-regulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant and Cell Physiology, 48, 606–614.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panda, S.K., Sahoo, L., Katsuhara, M. et al. Overexpression of Alternative Oxidase Gene Confers Aluminum Tolerance by Altering the Respiratory Capacity and the Response to Oxidative Stress in Tobacco Cells. Mol Biotechnol 54, 551–563 (2013). https://doi.org/10.1007/s12033-012-9595-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9595-7