Abstract
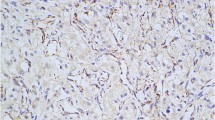
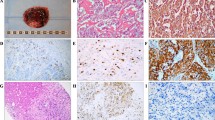
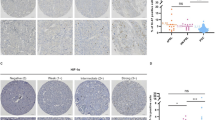
Although GATA3 has been recognized as a useful marker for mammary and urothelial carcinomas, there is large variation in GATA3 expression detected in pheochromocytoma (PC) and paraganglioma (PGL), from 90% to less than 5%. For GATA3 to be a useful diagnostic marker for PCCs/PGLs, the reasons for such discrepancy must be elucidated. Thus, we compared different immunohistochemistry protocols. Three protocols for GATA3 immunohistochemistry, including the use of an automated slide stainer or manual staining with an autoclave and EDTA buffer vs citric acid buffer, were compared. Whole sections of paraffin-embedded tumors, including 30 PCCs, 37 PGLs including 15 head and neck PGLs, 5 retroperitoneal PGLs, 17 urinary bladder PGLs, and 14 neuroblastoma group tumors, were examined and compared with mammary and urothelial carcinoma sections as positive controls. Using the automated slide stainer (Benchmark ULTRA; Ventana Medical Systems) with both buffers, mammary and urothelial carcinomas demonstrated strong GATA3 positivity; however, PCCs/PGLs showed negative or weak heterogeneous staining. Manual staining with an autoclave for antigen retrieval resulted in increased GATA3 immunoreactivity in all head and neck PGLs, all retroperitoneal PGLs, 88% of urinary PGLs, 17% of PCCs, and all neuroblastomas, except for ganglion cells. The normal adrenal medulla stained weakly and heterogeneously. In conclusions, immunohistochemistry for GATA3 in PCCs/PGLs requires stronger antigen retrieval than that in mammary and urinary carcinomas. This finding is especially important to consider if GATA3 is applied for the differential diagnosis of PGLs in unusual sites as supplemental data to the expression of catecholamine-synthesizing enzymes.



Similar content being viewed by others
References
Asa SL, Ezzat S, Mete O. The Diagnosis and Clinical Significance of Paragangliomas in Unusual Locations. J Clin Med 2018;7: 280–294. doi: https://doi.org/10.3390/jcm7090280
Yoon NK, Maresh EL, Shen D, Elshimali Y, Apple S, Horvath S, Mah V, Bose S, Chia D, Chang HR, Goodglick L Higher levels of GATA3 predict better survival in women with breast cancer. Hum Pathol 2010;41: 1794–1801.
Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127: 1041–1055.
Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, Lindeman GJ, Visvader JE Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9: 201–209.
Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138: 57–64.
Nonaka D, Wang BY, Edmondson D, Beckett E, Sun CC. A study of gata3 and phox2b expression in tumors of the autonomic nervous system. Am J Surg Pathol. 2013;37: 1236–1241.
Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, Langfort R, Waloszczyk P, Biernat W, Lasota J, Wang Z GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol. 2014;38: 13–22.
So JS, Epstein JI. GATA3 expression in paragangliomas: a pitfall potentially leading to misdiagnosis of urothelial carcinoma. Mod Pathol. 2013;26: 1365–1370.
Perrino CM, Ho A, Dall CP, Zynger DL. Utility of GATA3 in the differential diagnosis of pheochromocytoma. Histopathology. 2017;71: 475–479.
Weissferdt A, Kalhor N, Liu H, Rodriguez et al. Thymic neuroendocrine tumors (paraganglioma and carcinoid tumors): a comparative immunohistochemical study of 46 cases. Hum Pathol. 2014;45: 2463–2470.
Shi SR, Liu C, Taylor CR. Standardization of Immunohistochemistry for Formalin-fixed, Paraffin-embedded Tissue Sections Based on the Antigen-retrieval Technique: From Experiments to Hypothesis. J Histochem & Cytochem. 2007;55: 105–109.
Tsarovina K, Pattyn A, Stubbusch J, Müller F, van der Wees J, Schneider C, Brunet JF, Rohrer H Essential role of Gata transcription factors in sympathetic neuron development. Development 2004;131: 4775–4786.
Moriguchi T, Takako N, Hamada M, Maeda A, Fujioka Y, Kuroha T, Huber RE, Hasegawa SL, Rao A, Yamamoto M, Takahashi S, Lim KC, Engel JD Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 2006;133: 3871–3881.
Hoene V, Fischer M, Ivanova A, Wallach T, Berthold F, Dame C. GATA factors in human neuroblastoma: distinctive expression patterns in clinical subtypes. Br J Cancer. 2009;101: 1481–1489.
Peng H, Ke XX, Hu R, Yang L, Cui H, Wei Y. Essential role of GATA3 in regulation of differentiation and cell proliferation in SK-N-SH neuroblastoma cells. Mol Med Rep. 2015;11: 881–886
Tischler A.S., de Kriiger R.R., Gill A et al. Phaeochromocytoma. In Lloyd RV, Osamura RY, Kloppel G, Rosai J (eds) WHO Classification of Tumours of Endocrine Organs, 4th edition. IARC: Lyon 2017; pp: 183–189.
Kimura N, Capella C, DeLellis RA et al. Extra-adrenal paraganglioma. In Lloyd RV, Osamura RY, Kloppel G, Rosai J (eds) WHO Classification of Tumours of Endocrine Organs, 4th edition. IARC: Lyon 2017; pp: 190–195.
Osinga TE, Korpershoek E, de Krijger RR, Kerstens MN, Dullaart RP, Kema IP, van der Laan B, van der Horst-Schrivers A, Links TP Catecholamine-synthesizing enzymes are expressed in parasympathetic head and neck Paraganglioma tissue. Neuroendocrinology. 2015;101: 289–295.
Katagiri K, Shiga K, Ikeda A, Saito D, Oikawa S, Tshuchida K et al. Effective, Same-Day Preoperative Embolization and Surgical Resection of Carotid Body Tumors. Head Neck. 2019; 41: 3159–3167.
Sachs C, Allard C, Bellizzi A. Thyrosine hydroxylase is superior to GATA-3 for the diagnosis of pheochromocytoma/paraganglioma among neuroendocrine neoplasms. Mod Pathol. 2019;32 (suppl 2): 22–23.
Ordóñez NG. Value of GATA3 immunostaining in the diagnosis of parathyroid tumors. Appl Immunohistochem Mol Morphol. 2014;22 :756–761.
Acknowledgments
The authors thank Okuyama D, M.T. and Azuma M, M.T. for their dedication and cooperation regarding the immunohistochemical work.
Funding
This study was conducted as a part of the JRAS by a Research Grant from the Japan Agency for Medical Research and Development (AMED) under Grant Number JP19ek010935.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Noriko Kimura, Kiyoto Shiga, Kenichi Kaneko, Chiho Sugisawa, Takayuki Katabami, and Mitsuhide Naruse. The first draft of the manuscript was written by Noriko Kimura and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
Institutional REB approvals were obtained.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, N., Shiga, K., Kaneko, K. et al. The Diagnostic Dilemma of GATA3 Immunohistochemistry in Pheochromocytoma and Paraganglioma. Endocr Pathol 31, 95–100 (2020). https://doi.org/10.1007/s12022-020-09618-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-020-09618-1