Abstract
Purpose
To compare cardiovascular outcomes and rates of fractures and falls among patients with persistent brand-name versus generic L-thyroxine use.
Methods
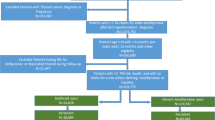
Retrospective, 1:1 propensity-matched longitudinal study using a national administrative claims database to examine adults (≥18 years) who initiated either brand or generic L-thyroxine between 2008 and 2018, censored at switch or discontinuation of L-thyroxine formulation or disenrollment from the health plan. Main outcome measures included rates of hospitalization for atrial fibrillation, myocardial infarction, congestive heart failure, stroke, spine and hip fractures, and rate of falls in the outpatient or inpatient setting. Hospitalizations for pneumonia were used as a negative control.
Results
195,046 adults initiated treatment with L-thyroxine between 2008 and 2017: 87% generic and 13% brand formulations. They were mostly women (76%), young (94.6% under age 65), white (66%), and 47% had baseline thyroid stimulating hormone levels between 4.5 and 9.9 mIU/L. Among 35,667 propensity-matched patients, there were no significant differences between patients treated with brand versus generic L-thyroxine in atrial fibrillation (HR 0.96, 0.58–1.60), myocardial infarction (HR 0.66, 0.39–1.14), congestive heart failure (HR 1.30, 0.78–2.16), stroke (0.72, 0.49–1.06), spine (HR 0.87, 0.38–1.99) and hip fractures (HR 0.86, 0.26–2.82), or fall outcomes (HR 1.02, 0.14–7.32). Hospitalization rates for pneumonia (used as negative control) did not differ between groups (HR 0.85, 0.61–1.19). There were no interactions between brand versus generic L-thyroxine, these outcomes, and thyroid cancer, age, or L-thyroxine dose subgroups.
Conclusions
We found no significant differences in cardiovascular outcomes and rates of falls and fractures for patients who filled brand versus generic L-thyroxine.

Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
R. Rodriguez-Gutierrez, S. Maraka, N.S. Ospina, V.M. Montori, J.P. Brito, Levothyroxine overuse: time for an about face? Lancet Diab. Endocrinol. (2017). https://doi.org/10.1016/S2213-8587(16)30276-5
E.D. Kantor, C.D. Rehm, J.S. Haas, A.T. Chan, E.L. Giovannucci, Trends in prescription drug use among adults in the United States from 1999-2012. Jama (2015). https://doi.org/10.1001/jama.2015.13766
M. Viswanathan, C.E. Golin, C.D. Jones et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann. Intern. Med. (2012). https://doi.org/10.7326/0003-4819-157-11-201212040-00538
J.S. Ross, S. Rohde, L. Sangaralingham et al. Generic and brand-name thyroid hormone drug use among commercially insured and medicare beneficiaries, 2007 Through 2016. J. Clin. Endocrinol. Metab. (2019). https://doi.org/10.1210/jc.2018-02197
N. Huo, L. Chen, A.U. Mishuk et al. Generic levothyroxine initiation and substitution among Medicare and Medicaid populations: a new user cohort study. Endocrine (2020). https://doi.org/10.1007/s12020-020-02211-w
American Thyroid Association, The Endocrine Society, and American Association of Clinical Endocrinologists. Joint Statement on the U.S. Food and Drug Administration’s Decision Regarding Bioequivalence of Levothyroxine Sodium (2021). https://www.endocrine.org/advocacy/position-statements/bioequivalence-of-sodium-levothyroxine
Endocrine Society. Bioequivalence of Sodium Levothyroxine (2008). https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf
A. Grossman, I. Feldhamer, J. Meyerovitch, Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur. J. Intern. Med. (2018). https://doi.org/10.1016/j.ejim.2017.11.010
P.N. Taylor, A. Iqbal, C. Minassian et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks: evidence from a large community-based study. JAMA Intern. Med. (2014). https://doi.org/10.1001/jamainternmed.2013.11312
R.C. Smallridge, L.R. Sangaralingham, R. Mwangi, F. Kusumoto, H. Van Houten, V. Bernet, Comparison of Incident Cardiovascular Event Rates Between Generic and Brand l-Thyroxine for the Treatment of Hypothyroidism. Mayo Clinic Proceedings (2019)
P.J. Wallace, N.D. Shah, T. Dennen, P.A. Bleicher, W.H. Crown, Optum Labs: building a novel node in the learning health care system. Health Affairs (2014). https://doi.org/10.1377/hlthaff.2014.0038
OptumLabs. Real world health care experiences from over 150 million unique individuals since 1993 (2015). https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf
J. Jonklaas, A. Bianco, A. Bauer et al., American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid (2014). https://doi.org/10.1089/thy.2014.0028
J.R. Desai, C.L. Hyde, S. Kabadi et al. Utilization of positive and negative controls to examine comorbid associations in observational database studies. Med. Care (2017). https://doi.org/10.1097/MLR.0000000000000640
R.A. Deyo, D.C. Cherkin, M.A. Ciol, Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. (1992). https://doi.org/10.1016/0895-4356(92)90133-8
D.F. McCaffrey, B.A. Griffin, D. Almirall, M.E. Slaughter, R. Ramchand, L.F. Burgette, A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat. Med. (2013). https://doi.org/10.1002/sim.5753
J.M. Carswell, J.H. Gordon, E. Popovsky, A. Hale, R.S. Brown, Generic and brand-name L-thyroxine are not bioequivalent for children with severe congenital hypothyroidism. J. Clin. Endocrinol. Metab. (2013). https://doi.org/10.1210/jc.2012-3125
Author contributions
J.P.B., J.S.R., L.S., N.D.S., and K.J.L. contributed to the study concept and design. J.P.B., J.S.R., Y.D., L.S., D.J.G., Y.Q., Z.W., X.Y., L.Z., N.D.S., and K.J.L. contributed to the development of the study protocol. Y.D. and L.S. were responsible for conducting the data analysis for this study. All authors contributed to the data interpretation. All authors critically reviewed the manuscript.
Funding
This project was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award [Center of Excellence in Regulatory Science and Innovation grant to Yale University and Mayo Clinic, U01FD005938]. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.S.R. has received research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Medical Device Innovation Consortium as part of the National Evaluation System for Health Technology (NEST), from the Agency for Healthcare Research and Quality (R01HS022882), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R01HS025164), and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International and to establish the Collaboration for Research Integrity and Transparency (CRIT) at Yale. N.D.S. has received research support through Mayo Clinic from the Centers of Medicare and Medicaid Innovation, from the Agency for Healthcare Research and Quality (R01HS025164; R01HS025402; R03HS025517; U19HS024075), from the National Heart, Lung and Blood Institute of the National Institutes of Health (NIH) (R56HL130496; R01HL131535), National Science Foundation, and from the Patient Centered Outcomes Research Institute (PCORI). K.J.L. receives support from the Centers of Medicare and Medicaid Services (CMS) and the National Institute on Aging and the American Federation of Aging Research through the Paul Beeson Career Development Award (K23AG048359). R.C.S. receives support from the Alfred D. and Audrey M. Petersen Professorship in Cancer Research. D.J.G., Y.Q., Z.W., and L.Z. are employed by the US Food and Drug Administration. The remaining authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix Table 1
Appendix Table 1
Clinically relevant medications that may affect thyroid function |
• Antithyroid Medications (PTU, Propylthiouracil, 6-N Propylthiouracil, Methimazole, Felimazole, Northyx, Tapazole, Thiamazole, Carbimazole, Benzylthiouracil, Methylthiouracil) |
• Lithium carbonate |
• Amiodarone hydrochloride |
• Phenytoin |
• Interferon alfa |
• Interleukin 2 |
• Gefitinib |
• Erlotinib |
• Sorafenib |
• Sunitinib |
• Dasatinib |
• Lenvatinib |
• Imatinib |
• Cabozantinib |
• Vandetanib |
Rights and permissions
About this article
Cite this article
Brito, J.P., Ross, J.S., Deng, Y. et al. Cardiovascular outcomes and rates of fractures and falls among patients with brand-name versus generic L-thyroxine use. Endocrine 74, 592–602 (2021). https://doi.org/10.1007/s12020-021-02779-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-021-02779-x