Abstract
Introduction
Clinical guidelines recommend levothyroxine as the standard of care for hypothyroidism and that patients should be treated with a consistent preparation of synthetic levothyroxine without switching among formulations. This study examines the likelihoods of negative clinical outcomes between continuous users of Synthroid® (AbbVie, Inc.) and patients who switch from Synthroid® to an alternative formulation of levothyroxine.
Methods
This retrospective cohort analysis utilized data from Optum Clinformatics™ DataMart covering May 1, 2000 to March 30, 2016. After 6 months of consistent use of Synthroid®, patients were categorized as continuous users or as switchers (by filling a prescription for an alternative formulation). Key outcomes included the likelihood of a thyroid-stimulating hormone (TSH) laboratory value out of a guideline recommended range and/or an adverse clinical composite endpoint identified by ICD codes in the patient’s claims data over the following 2 years for any of the following: chronic kidney disease, depression, fatigue, heart failure, hyperlipidemia, hypertension, or obesity. Individual components of the composite endpoint were also examined. Outcomes were analyzed using multivariable logistic models on propensity score matched cohorts. Analyses controlled for patient characteristics using SAS 9.4 software. Chi-square and t tests were employed and P < 0.05 was pre-specified as statistically significant.
Results
Propensity score matching resulted in a sample of 9925 continuous users and 9925 switchers. Switchers were significantly more likely than continuers to have a TSH laboratory value out-of-range in the post-period [odds ratio (OR) 1.15; 95% confidence interval (CI) (1.08–1.23)]. Switchers were also more likely to have the composite clinical endpoint [OR 1.23; CI (1.12–1.37)] and to have individual diagnoses of chronic kidney disease, depression, fatigue, hypertension, or obesity in the post-period.
Conclusions
Results of this large retrospective study over an extended time horizon support clinical guideline recommendations that switching among alternative formulations of synthetic levothyroxine should generally be avoided. Continuous use of Synthroid® was associated with a significantly higher likelihood of maintaining the TSH laboratory value within a guideline recommended range and a significantly lower likelihood of being diagnosed with adverse clinical outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Hypothyroidism is common in the USA and can be clinically overlooked but, nevertheless, is associated with a variety of adverse clinical outcomes. |
Clinical guidelines recommend levothyroxine (LT4) as the standard of care for hypothyroidism and that patients should be treated with a consistent preparation of synthetic levothyroxine without switching among formulations. |
This study examined clinical outcomes (TSH laboratory values out of recommended ranges and diagnoses of comorbidities) between two cohorts of patients with hypothyroidism, one treated continuously with Synthroid® and the other that switched to an alternative LT4 formulation. |
What was learned from the study? |
Among insured adults in the USA with hypothyroidism who had initial stable treatment with Synthroid®, people who were switched to an alternative LT4 formulation tended to be older, more likely to be male, and less likely to have had a visit to an endocrinologist’s office than those who were treated continuously with Synthroid®. |
Switching to an alternative LT4 formulation was associated with a higher likelihood of a TSH laboratory value outside of the target range in the post-period as compared to continuous use of Synthroid®. |
Compared to continuous treatment, switching was associated with higher likelihoods of being diagnosed with a number of negative clinical outcomes: chronic kidney disease, depression, fatigue, hypertension, or obesity as well as a composite clinical endpoint. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13078886.
Introduction
An estimated 4.6% [1] of the US population have hypothyroidism, an endocrine disorder characterized by insufficient generation of endogenous thyroid hormone (thyroxine) and, to a lesser extent, triiodothyronine. Hashimoto’s (autoimmune) thyroiditis is the most common cause of hypothyroidism in the USA [2]. Hypothyroidism can be clinically overlooked as it may present with nonspecific complaints or symptoms that may be attributed to other conditions [3]. Known associations between hypothyroidism and other serious comorbidities include depression [4, 5], obesity [6, 7], fatigue [8, 9], hyperlipidemia [3, 10, 11], hypertension [12, 13], chronic kidney disease (CKD) [14], and heart failure [3].
The American Association of Clinical Endocrinologists (AACE) and the American Thyroid Association (ATA) have issued joint guidelines recommending levothyroxine (LT4) as the standard of care for the management of hypothyroidism and that the dose be adjusted so as to maintain thyroid-stimulating hormone (TSH) levels within a target range (the range being dependent upon personal characteristics of the patient such as age and pregnancy status.) [15]. While there are various formulations of LT4 that are available for use in clinical practice, current recommendations encourage the use of a consistent l-thyroxine preparation for individual patients to minimize variability from refill to refill [15]. This recommendation was based on several factors, including LT4’s narrow therapeutic index, a lack of sufficient data about natural thyroid formulations, and the uniqueness of the various tablet formulations of LT4 [15]. Studies of hypothyroidism treatment in clinical practice have examined the concordance of TSH outcomes achieved with the guideline recommendations. For instance, one study evaluated pregnant women with hypothyroidism treated with LT4 (N = 2340) and found that only 52.6% of pregnant women achieved TSH levels within the trimester-specific target range [16]. Among older adults diagnosed with varying degrees of hypothyroidism (N = 4025), a substantial proportion (28.0%) were not receiving levothyroxine therapy and, of those who did receive such therapy (N = 2899), 32.9% were not being monitored to determine whether the dosage was appropriate [17]. Examination of the consequences of switching among LT4 formulations has shown that such switching was associated with changes in TSH levels in children with hypothyroidism [18] and, in other studies, higher medical costs [19, 20]. More generally, only about 50% of patients with hypothyroidism adhere to LT4 prescription regimens during the first year of treatment [21] and higher medical costs have also been associated with nonadherence [22]. However, little research has explored how LT4 switching may affect the likelihood of having specific clinical outcomes.
The purpose of the present study was to compare the clinical outcomes of two cohorts of patients with hypothyroidism treated with LT4 over a 2-year follow-up period: those who switched from Synthroid® (AbbVie, Inc.) to an alternative formulation of LT4 and those who were continuous users of Synthroid®. The outcomes of interest included the likelihood of having an out-of-range TSH level and the odds of being diagnosed with additional comorbidities.
Methods
This observational, matched cohort study used data spanning the time frame from May 1, 2000 through March 30, 2016 from the Optum Clinformatics™ database. The database contains information on over 150 million individuals and laboratory results for over 30 million people. Before release, the data were verified, adjudicated, and adjusted. Data elements include patient demographics, inpatient and outpatient services, and outpatient prescription drugs. The information is fully de-identified and HIPAA compliant. Given the retrospective nature of the study design and de-identified data, the study was exempt from internal review board evaluation.
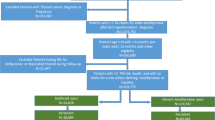
For inclusion in the study, patients were required to initially have compliant use of Synthroid® for a 6-month lead-in period, where compliant use was defined as receipt of at least 146 days’ supply of Synthroid® (i.e., greater than 80% medication possession ratio) for the 6-month lead-in period with no filling of prescriptions for any other LT4 formulation. In the subsequent 6-month identification period, patients were defined as continuous users of Synthroid® or switchers from Synthroid® to an alternative LT4 formulation. Specifically, continuous users filled at least one prescription for Synthroid® and did not fill any prescriptions of non-Synthroid® formulations of LT4, while switchers filled at least one prescription for a non-Synthroid® formulation during the identification period. The non-Synthroid formulations considered in the study included Levothroid, Levoxyl, Tirosint, Unithroid, and generic levothyroxine. For continuous users, the index date was defined as the date of first fill of a Synthroid® prescription during the identification period, while for switchers the index date was defined as the date of first fill for non-Synthroid® LT4 prescription during the identification period. The post-period was defined as the time from the index date through 2 years following the index date. Figure 1 presents the study design.
In addition to being identified as a continuous user or switcher, patients were required to have received at least one diagnosis of hypothyroidism (ICD-9-CM codes 243, 244.0, 244.1, 244.8, 244.9 or ICD-10-CM of E030, E031, E038, E039, E890) at some point from the start of the lead-in period through the end of the post-period (i.e., the study period), to have at least one TSH laboratory test result recorded during the last 6 months of the post-period, and to have had continuous insurance coverage over the study period. Patients were excluded if they were identified as pregnant (ICD-9-CM of 630.xx-679.xx, V22.xx, V23.xx or ICD-10-CM of Oxxxx, Z33xx, Z34xx) or if a diagnosis of thyroid cancer (ICD-9-CM of 193 or ICD-10-CM of C73), iodine hypothyroidism, or other iatrogenic hypothyroidism (ICD-9-CM of 244.2, 244.3 or ICD-10-CM of E00xx, E01xx, E02xx, E032) was included in the patient’s record at any time over the study period. In addition, patients were excluded if they filled a prescription for liothyronine or a liothyronine–levothyroxine combination therapy during the study period or if they were younger than 18 years old at the index date. Figure 2 shows how these inclusion/exclusion criteria affected sample size.
Inclusion/exclusion criteria and sample size. Study period defined from the beginning of the lead-in period through the end of the 2-year post-period (see Fig. 1)
Given the above cohort, a 1:1 nearest neighbor, greedy matching algorithm without replacement was applied to match continuous users and switchers on the basis of propensity scores. The propensity score model estimated the probability that a patient would switch therapy while controlling for patient-level characteristics. Differences in post-period outcomes between the propensity score matched groups were then examined using multivariable logistic regressions.
The key outcomes of interest were TSH laboratory values that were not within the range recommended by expert bodies and, also, negative clinical outcomes. The primary recommended TSH laboratory value range (0.45–4.12 mIU/L) was based upon AACE/ATA guidelines (2012) while an alternative TSH range, (0.4–4.0 mIU/L) based upon ATA task force guidelines (2014), was used in a sensitivity analysis. A negative clinical outcome was defined as a composite endpoint, operationalized as receipt of a diagnosis of at least one of the following: chronic kidney disease (CKD), depression, fatigue, heart failure, hyperlipidemia, hypertension, or obesity, during the post-period. In addition, the likelihoods of an individual negative clinical outcome in the post-period for any of the above conditions were also examined.
Both the propensity score model and the logistic regressions controlled for patient characteristics and general health status in the 6 months prior to the index date (i.e., the pre-period). Specifically, the analyses controlled for patient age, sex, region of residence, type of insurance coverage, pre-period Charlson Comorbidity Index (CCI) score, and visits to an endocrinologist. In addition, the propensity score model also controlled for the dose of Synthroid® and the average copayment for Synthroid® in the lead-in period, while the models that examined outcomes controlled for index LT4 prescription dose and copayment. Multivariable logistic models that examined patient outcomes also included a lagged dependent variable to control for prior presence of the condition of interest. As exploratory analyses, the associations between the key outcomes and the number of switches among LT4 medications in the post-period were examined. In these analyses, a subsequent switch could have been to any of the other LT4 formulations, including a switch back to Synthroid®.
All analyses were conducted using SAS, Version 9.4 (Cary, NC), and a P value less than 0.05 was considered, a priori, to be statistically significant.
Results
There were 25,363 continuous users and 10,080 switchers included in this study (Fig. 2). After 1:1 propensity score matching, 9925 patients remained in each cohort. Table 1 shows characteristics of continuous users and switchers both before and after propensity score matching. Prior to the match, there were statistically significant differences between continuous users and switchers, with switchers being statistically significantly older, more likely to be male, and less likely to have visited an endocrinologist in the pre-period. Furthermore, switchers were statistically significantly more likely to have pre-existing CKD, depression, hyperlipidemia, hypertension, or obesity. After propensity score matching, some significant pre-period differences between continuous users and switchers remained, but numerical differences between the two groups generally were reduced. After the match, switchers were more likely to have visited an endocrinologist. CKD and obesity also continued to be statistically significantly different between switchers and continuous users. There were no statistically significant differences between the cohorts in having a TSH laboratory value out of range during the pre-period.
Figure 3 shows the association between switching LT4 formulations and having a TSH value outside of range. Both the main and sensitivity analyses indicate that switching was associated with a higher likelihood of having a TSH value outside of the target range in the post-period compared continuous users. Specifically, under the 2012 guidelines, switching was associated with a 15% higher likelihood of an out-of-range TSH laboratory result compared to continuous use of Synthroid® [odds ratio (OR) 1.15; 95% confidence interval (CI) (1.08–1.23)]. Using the 2014 guidelines, switching was associated with a 13% higher likelihood of a TSH laboratory value out-of-range according to these updated guidelines [OR 1.13; CI (1.04–1.20)].
Associations of switching and TSH Labs out of range (during post-period). Results from multivariable logistic regressions which controlled for patient characteristics (age, sex, region, insurance type) index dose, index copay, pre-period CCI, pre-period visit to endocrinologist, and lagged dependent variable
Figure 4 shows the associations between switching and clinical outcomes. Results illustrate that after controlling for patient demographics, index dosing, copayment for LT4, pre-period CCI score, pre-period visit to an endocrinologist, and the prior existence of a condition of interest, switching was associated with a higher likelihood of the negative clinical outcome in the 2-year post-period compared to continuous use of Synthroid®. Specifically, switching was associated with a 23% higher likelihood of the negative composite endpoint compared to continuous use [OR 1.23; CI (1.12–1.37)]. When examining each of the individual components of the composite endpoint, switching was associated with a higher likelihood of being diagnosed with CKD [OR 1.42; CI (1.10–1.83)], depression [OR 1.14; CI (1.04–1.26)], fatigue [OR 1.16; CI (1.08–1.24)], hypertension [OR 1.12; CI (1.03–1.22)], or obesity [OR 1.36; CI (1.21–1.52)]. However, there were no statistically significant differences between the two groups when examining the likelihood of being diagnosed with heart failure or hyperlipidemia in the post-period.
Associations of switching and clinical diagnoses (during post-period). Results from multivariable logistic regressions which controlled for patient characteristics (age, sex, region, insurance type) index dose, index copay, pre-period CCI, pre-period visit to endocrinologist, and lagged dependent variable
While Figs. 3 and 4 compare outcomes between continuous users and switchers, Table 2 presents the associations between outcomes and the number of switches. As Table 2 reveals, the impact of switching only once is similar to that of switching in general, with all clinical outcomes examined except heart failure and hyperlipidemia being associated with a significantly higher occurrence among patients who switched once in the post-period relative to continuous users. In contrast, switching three or more times in the post-period was associated with a higher likelihood of all of the outcomes examined. In addition, the odds ratios associated with switching three or more times were numerically greater than the odds ratios associated with switching only once, indicating that three or more switches increased the likelihood of any adverse clinical outcome examined. As an additional test of the robustness of the results, all analyses were re-conducted with the addition of a variable—year of the index date—to control for cohort effect (results not shown). Results of these last analyses were generally not sensitive to this alternative specification with these exceptions: (1) switching was no longer associated with increased likelihoods of post-period diagnoses of CKD or fatigue; and (2) switching was associated with a significantly increased likelihood of a post-period diagnosis of hyperlipidemia.
Discussion
Using real-world data, this study examined the clinical consequences of switching from Synthroid® to an alternative formulation of LT4. The outcomes of interest in this study included the likelihood of having out-of-range TSH laboratory results or the prevalence of clinical diagnoses in continuous users of Synthroid® and switchers. The findings indicated that switchers are more likely to have negative clinical outcomes relative to continuous users of Synthroid®. For instance, patients with hypothyroidism and prior compliant use of Synthroid® over a 6-month period and who then switched to an alternative LT4 formulation were more likely to have TSH laboratory values outside the recommended target levels for TSH than were patients who continued filling prescriptions for Synthroid®. Further, relative to the continuous users of Synthroid®, those who switched to another LT4 formulation were 23% more likely to have met the negative composite endpoint and had higher likelihoods of having an individual diagnosis of five of the seven comorbidities included in the analysis. A caveat is that the propensity score model did not work perfectly to balance the two cohorts on all the patient characteristics, in particular the proportions of patient with CKD and obesity. In evaluating the increased risk associated with switching that was observed for these two clinical outcomes, it is important to recall the statistically significant differences that existed at baseline. Further, obesity has been recognized as a condition that has historically been unreliably recorded in claims data which may lead to bias in the current study’s result for this condition [23].
Most patients switched only once. However, when the number of switches in the post-period was greater than or equal to three, the exploratory analyses showed that the odds of a negative clinical outcome were numerically higher when compared to one switch. Furthermore, the results for all individual comorbidities examined in patients who switched three or more times were statistically significant when compared against continuous use of Synthroid®. Regardless of the number of switches, switchers were also more likely to have TSH levels outside the recommended target range. In sum, these findings support clinical guidelines which advise that patients with hypothyroidism should continue therapy with the same synthetic LT4 formulation and avoid switching among LT4 formulations, when possible [15].
The present study may complement previous research on the health economic consequences of switching among LT4 formulations in patients with hypothyroidism. Healthcare providers and payers may determine to switch users of Synthroid® to alternative generic LT4 formulations in order to lower the costs of providing treatment [19]. However, studies have shown that the lower drug acquisition costs are more than offset by increases in other cost categories (e.g., hypothyroidism-related non-drug medical costs) such that total treatment costs are higher for switchers than continuous users of Synthroid® [19, 20]. The present study offers a possible explanation for those cost findings in that the presence of comorbid conditions and TSH excursions may require additional clinical follow-up and additional healthcare costs associated with management of comorbidities.
Limitations
Claims data research has general limitations. Insurance claims provide images of the financial transactions between facilities (outpatient, inpatient, and pharmacy) and payers (insurance companies), and these images reflect but do not fully document the interactions between patients and physicians. For instance, the presence of a diagnosis code on a medical claim does not guarantee the positive presence of a disease; a diagnosis may have been coded incorrectly or included as a rule-out criterion. Furthermore, a richer dataset would have allowed for additional adjustment and interpretation of results. For instance, pill-taking behaviors, use of nutritional supplements, or patient-reported outcomes are just a few examples of data that are not captured in insurance claims data. Also, while switching behavior, including the number of switches, was captured in the data, the reason(s) for switching among LT4 formulations was not available. In addition to the general limitations of claims-based research, there were several limitations specific to this study. For instance, the switching examined was not restricted to LT4 formulations that were A/B rated to Synthroid® but, rather, considered all switches among LT4 formulations which were found in these data that are reflective of clinical practice. Further, the analyses may underestimate the true incidence of switching as the study only evaluated patients who were compliant users of Synthroid® during the lead-in period. The reference range for TSH values is a population-based reference that varies according to which specific assay manufacturer is used and the same method may not produce the same results between assay manufacturers because of the multiple isomers of TSH. The clinical outcome of interest, i.e., a diagnosis in the post-period, may not have represented an incident case; for some individuals the diagnosis was present before the switch. To control for the presence of ICD codes at baseline before switch, a lagged dependent variable of diagnosis was included in statistical models. The analyses focused on individuals who were continuously insured for a period of at least 2 years after they initiated treatment with Synthroid®, and this population may not be generalizable to the population of all patients prescribed Synthroid®. Finally, the analyses focused on associations between treatment patterns and outcomes; no causal inferences can be drawn from associative results.
Conclusions
Study findings support the notion that hypothyroidism treatment outcomes may improve if providers and patients can reduce the amount of switching among LT4 medications and reinforces the importance of utilizing a consistent LT4 formulation to treat patients with hypothyroidism. As such, this study ties together and supports previous research on switching behavior, medical resource use, and costs among patients with hypothyroidism. Future work should examine if these results hold if switching is restricted to formulations that are A/B rated to Synthroid®. Also, since only well-insured patients were included in these analyses, future research that includes uninsured individuals with hypothyroidism may provide a more complete picture of patterns of care and health outcomes for patients with hypothyroid dysregulation.
References
Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–99.
Vanderpump MPJ. The epidemiology of thyroid disease. Br Med Bull. 2011;99:39–51.
Roberts CGP, Ladenson PW. Hypothyroidism. Lancet. 2004;363(9411):793–803.
Duntas LH, Maillis A. Hypothyroidism and depression: salient aspects of pathogenesis and management. Minerva Endocrinol. 2013;38(4):365–77.
Hage MP, Azar ST. The link between thyroid function and depression. J Thyroid Res. 2012;2012:590648.
Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316(2):165–71.
Sami A, Iftekhar MF, Rauf MA, Sher A. Subclinical hypothyroidism among local adult obese population. Pak J Med Sci. 2018;34(4):980–3.
Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid. 2014;24(12):1670–751.
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Hypothyroidism (underactive thyroid). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/endocrine-diseases/hypothyroidism. Accessed 9 Oct 2018.
Mansourian AR. The state of serum lipids profiles in sub-clinical hypothyroidism: a review of the literature. Pak J Biol Sci. 2010;13(11):556–62.
Rastgooye Haghi A, Solhjoo M, Tavakoli MH. Correlation between subclinical hypothyroidism and dyslipidemia. Iran J Pathol. 2017;12(2):106–11.
Cai Y, Ren Y, Shi J. Blood pressure levels in patients with subclinical thyroid dysfunction: a meta-analysis of cross-sectional data. Hypertens Res. 2011;34(10):1098–105.
Stabouli S, Papakatsika S, Kotsis V. Hypothyroidism and hypertension. Expert Rev Cardiovasc Ther. 2010;8(11):1559–65.
Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes. 2016;23(5):407–15.
Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18(6):988–1028.
Lage MJ, Vora J, Hepp Z, Espaillat R. Levothyroxine treatment of pregnant women with hypothyroidism: retrospective analysis of a US Claims Database. Adv Ther. 2020;37(2):933–45.
Lage MJ, Vora J, Hepp Z, Espaillat R. Prevalence and treatment of hypothyroidism: a retrospective analysis of older Americans. In: Poster presented at: ATA Annual Meeting; Victoria, BC, Canada; 2017.
Carswell JM, Gordon JH, Popovsky E, Hale A, Brown RS. Generic and brand-name l-thyroxine are not bioequivalent for children with severe congenital hypothyroidism. J Clin Endocrinol Metab. 2013;98(2):610–7.
Katz M, Scherger J, Conard S, Montejano L, Chang S. Healthcare costs associated with switching from brand to generic levothyroxine. Am Health Drug Benefits. 2010;3(2):127–34.
Khandelwal N, Johns B, Hepp Z, Castelli-Haley J. The economic impact of switching from Synthroid for the treatment of hypothyroidism. J Med Econ. 2018;21(5):518–24.
Hepp Z, Wyne K, Manthena SR, Wang S, Gossain V. Adherence to thyroid hormone replacement therapy: a retrospective, claims database analysis. Curr Med Res Opin. 2018;34(9):1673–8.
Hepp Z, Lage MJ, Espaillat R, Gossain VV. The association between adherence to levothyroxine and economic and clinical outcomes in patients with hypothyroidism in the US. J Med Econ. 2018;21(9):912–9.
Mocarski M, Tian Y, Smolarz BG, McAna J, Crawford A. Use of International Classification of Diseases, Ninth Revision codes for obesity: trends in the united states from an electronic health record-derived database. Popul Health Manag. 2018;21(3):222–30.
Acknowledgements
Funding
AbbVie, Inc. provided financial support for this study, the Open Access Fee and the Rapid Service Fee. AbbVie participated in the study design, research, data collection, analysis, and interpretation of data, writing, reviewing, and approving the publication.
Medical Writing and/or Editorial Assistance
The authors would like to thank Michael Treglia of HealthMetrics Outcomes Research, LLC for assistance in preparing the manuscript. AbbVie, Inc. provided funding for this work.
Authorship
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. No honoraria or payments were made for authorship.
Authorship Contributions
James V. Hennessey was instrumental in co-developing the idea for this study, co-developed the study questions to be asked, monitored initial and subsequent results and contributed significantly to manuscript preparation, editing and final review. Ramon Espaillat and Maureen J. Lage participated in the data collection. All authors participated in the study design, analysis and interpretation of data, and writing, reviewing and approving the publication.
Disclosures
Ramon Espaillat, Yinghui Duan and Seema Soni-Brahmbhatt are employees of AbbVie, Inc. and may own shares of AbbVie stock. Maureen J. Lage is Managing Member of HealthMetrics Outcomes Research, LLC which was compensated for work on this project. James V. Hennessey has previously served as an academic consultant to AbbVie, developing educational material in regard to thyroid hormone bioequivalence issues, technical regulatory filing advise and other clinical study designs. He has received no compensation for his contributions to this project. Peter Singer has previously served as consultant to AbbVie, Inc. and reports no conflicts on this research.
Compliance with Ethics Guidelines
The data used in this study are fully de-identified and HIPAA compliant. Given the retrospective nature of the study design and the de-identified data, the study was exempt from ethics board approval.
Data Availability
The data that support the findings of this study are available from Optum Clinformatics® but restrictions apply to the availability of these data, which were used under license for the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hennessey, J.V., Espaillat, R., Duan, Y. et al. The Association Between Switching from Synthroid® and Clinical Outcomes: US Evidence from a Retrospective Database Analysis. Adv Ther 38, 337–349 (2021). https://doi.org/10.1007/s12325-020-01537-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01537-1