Abstract
Purpose
Studies showed that TLR4 knockout (TLR4KO) could mitigate obesity and insulin resistance induced by high-fat diet in rats. In this study, we further investigated the effects of TLR4KO on islet function and pancreatic proteomics in obese rats by high-fat diet.
Methods
PA-induced lipotoxicity β-cells, SD and TLR4KO rats were used in this study. iTRAQ was used to screen out meaningful differential proteins.The protein expression level was evaluated by Western blotting; the cell apoptosis was detected by TUNEL assay.
Results
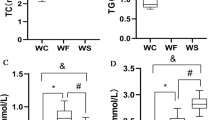
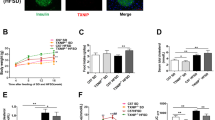
TLR4KO could reduce inflammatory and regulate body composition in obese rats, and improve β-cells function. The quantitative analysis of protein revealed that TLR4KO rebalanced proteomics disorders in pancreas of obese rats. In addition, the pathways involved in differential proteins were mainly metabolic pathways, arachidonic acid metabolism, ECM–receptor interaction, pancreatic secretion, PI3K-Akt signaling pathway, and FoxO signaling pathway. Further analysis of protein–protein interaction (PPI) revealed that Stk39 and Ass1 interacting through Mapk14-Ywhae were node proteins and participated in inflammatory response, carboxylic acid metabolic process, and small molecule metabolic process. In vitro experiments we confirmed that silencing TLR4 can inhibit PA-induced β-cell apoptosis, insulin secretion disorders, and increase Ass1 expression. While, overexpression of Ass1 in β-cell inhibited PA or LPS-induced β-cell damage.
Conclusions
Our study confirmed that TLR4KO could improve dysfunction of β-cell, and the underlying mechanism might be involved in ebalancing proteomics disorders in pancreas, affecting the expression of Ass1.






Similar content being viewed by others
References
X. Liu, X. Zeng, X. Chen, R. Luo, L. Li, C. Wang, J. Liu, J. Cheng, Y. Lu, Y. Chen, Oleic acid protects insulin-secreting INS-1E cells against palmitic acid-induced lipotoxicity along with an amelioration of ER stress. Endocrine 64, 512–524 (2019)
I.M. Taha, A.A.M. Abdu, E.G.E.M. Abd, Expression of toll-like receptor 4 and its connection with type 2 diabetes mellitus. Cell. Mol. Biol. (Noisy-le.-Gd.) 64, 15–20 (2018)
C. Tao, W.L. Holland, Q.A. Wang, M. Shao, L. Jia, K. Sun, X. Lin, Y.C. Kuo, J.A. Johnson, R. Gordillo, J.K. Elmquist, P.E. Scherer, Short-term versus long-term effects of adipocyte toll-like receptor 4 activation on insulin resistance in male mice. Endocrinology 158, 1260–1270 (2017)
L.A. Videla, G. Tapia, R. Rodrigo, P. Pettinelli, D. Haim, C. Santibañez, A.V. Araya, G. Smok, A. Csendes, L. Gutierrez, J. Rojas, J. Castillo, O. Korn, F. Maluenda, J.C. Díaz, G. Rencoret, J. Poniachik, Liver NF-kappaB and AP-1 DNA binding in obese patients. Obes. (Silver Spring) 17, 973–979 (2009)
D.S. Razolli, J.C. Moraes, J. Morari, R.F. Moura, M.A. Vinolo, L.A. Velloso, TLR4 expression in bone marrow-derived cells is both necessary and sufficient to produce the insulin resistance phenotype in diet-induced obesity. Endocrinology 156, 103–113 (2015)
J. Li, L. Chen, Y. Zhang, W.J. Zhang, W. Xu, Y. Qin, J. Xu, D. Zou, TLR4 is required for the obesity-induced pancreatic beta cell dysfunction. Acta Biochim. Biophys. Sin. 45, 1030–1038 (2013)
X. Shen, L. Yang, S. Yan, H. Zheng, L. Liang, X. Cai, M. Liao, Fetuin A promotes lipotoxicity in β cells through the TLR4 signaling pathway and the role of pioglitazone in anti-lipotoxicity. Mol. Cell. Endocrinol. 412, 1–11 (2015)
H.Q. Liu, Y. Qiu, Y. Mu, X.J. Zhang, L. Liu, X.H. Hou, L. Zhang, X.N. Xu, A.L. Ji, R. Cao, R.H. Yang, F. Wang, A high ratio of dietary n-3/n-6 polyunsaturated fatty acids improves obesity-linked inflammation and insulin resistance through suppressing activation of TLR4 in SD rats. Nutr. Res 33, 849–858 (2013)
J.J. Cha, Y.Y. Hyun, M.H. Lee, J.E. Kim, D.H. Nam, H.K. Song, Y.S. Kang, J.E. Lee, H.W. Kim, J.Y. Han, D.R. Cha, Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology 154, 2144–2155 (2013)
L. Jia, C.R. Vianna, M. Fukuda, E.D. Berglund, C. Liu, C. Tao, K. Sun, T. Liu, M.J. Harper, C.E. Lee, S. Lee, P.E. Scherer, J.K. Elmquist, Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Commun. 5, 3878 (2014)
X. Hu, S. Liu, X. Liu, J. Zhang, Y. Liang, Y. Li, DPP-4 (CD26) inhibitor sitagliptin exerts anti-inflammatory effects on rat insulinoma (RINm) cells via suppressing NF-κB activation. Endocrine 55, 754–763 (2017)
Q. Xie, S. Zhang, C. Chen, J. Li, X. Wei, X. Xu, F. Xuan, N. Chen, T. Pham, N. Qin, J. He, F. Ye, W. Huang, R. Huang, Q. Wen, Protective effect of 2-dodecyl-6-methoxycyclohexa-2, 5-diene-1, 4-dione, isolated from averrhoa carambola l., against palmitic acid-induced inflammation and apoptosis in Min6 cells by inhibiting the TLR4-MyD88-NF-κB signaling pathway. Cell. Physiol. Biochem. 39, 1705–1715 (2016)
J. Ke, R. Wei, F. Yu, J. Zhang, T. Hong, Liraglutide restores angiogenesis in palmitate-impaired human endothelial cells through PI3K/Akt-Foxo1-GTPCH1 pathway. Peptides 86, 95–101 (2016)
S. Camandola, M.P. Mattson, Toll-like receptor 4 mediates fat, sugar, and umami taste preference and food intake and body weight regulation. Obes. (Silver Spring) 25, 1237–1245 (2017)
L. Guo, C.H. Chen, L.L. Zhang, X.J. Cao, Q.L. Ma, P. Deng, G. Zhu, C.Y. Gao, B.H. Li, Y. Pi, Y. Liu, Z.C. Hu, L. Zhang, Z.P. Yu, Z. Zhou, J.C. Li, IRAK1 mediates TLR4-induced ABCA1 downregulation and lipid accumulation in VSMCs. Cell Death Dis. 6, e1949 (2015)
M. Wang, S. Li, F. Wang, J. Zou, Y. Zhang, Aerobic exercise regulates blood lipid and insulin resistance via the toll-like receptor 4-mediated extracellular signal-regulated kinases/AMP-activated protein kinases signaling pathway. Mol. Med Rep. 17, 8339–8348 (2018)
D. Pal, S. Dasgupta, R. Kundu, S. Maitra, G. Das, S. Mukhopadhyay, S. Ray, S.S. Majumdar, S. Bhattacharya, Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 18, 1279–1285 (2012)
C. Sathishkumar, P. Prabu, M. Balakumar, R. Lenin, D. Prabhu, R.M. Anjana, V. Mohan, M. Balasubramanyam, Augmentation of histone deacetylase 3 (HDAC3) epigenetic signature at the interface of proinflammation and insulin resistance in patients with type 2 diabetes. Clin. Epigenetics 8, 125 (2016)
H. Inoue, J. Shirakawa, Y. Togashi, K. Tajima, T. Okuyama, M. Kyohara, Y. Tanaka, K. Orime, Y. Saisho, T. Yamada, K. Shibue, R.N. Kulkarni, Y. Terauchi, Signaling between pancreatic β cells and macrophages via S100 calcium-binding protein A8 exacerbates β-cell apoptosis and islet inflammation. J. Biol. Chem. 293, 5934–5946 (2018)
G.M. Tannahill, L.A. O’Neill, The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3. FEBS Lett. 585, 1568–1572 (2011)
C. Fava, E. Danese, M. Montagnana, M. Sjögren, P. Almgren, G. Engström, P. Nilsson, B. Hedblad, G.C. Guidi, P. Minuz, O. Melander, Serine/threonine kinase 39 is a candidate gene for primary hypertension especially in women: results from two cohort studies in Swedes. J. Hypertens. 29, 484–491 (2011)
A. Persu, L. Evenepoel, Y. Jin, A. Mendola, G. Ngueta, W.Y. Yang, D. Gruson, S. Horman, J.A. Staessen, M. Vikkula, STK39 and WNK1 are potential hypertension susceptibility genes in the BELHYPGEN cohort. Med. (Baltim.) 95, e2968 (2016)
I. Torre-Villalvazo, L.G. Cervantes-Pérez, L.G. Noriega, J.V. Jiménez, N. Uribe, M. Chávez-Canales, C. Tovar-Palacio, B.A. Marfil-Garza, N. Torres, N.A. Bobadilla, A.R. Tovar, G. Gamba, Inactivation of SPAK kinase reduces body weight gain in mice fed a high-fat diet by improving energy expenditure and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 314, E53–53E65 (2018)
K.J.P. Ten, A. Sotiriou, S. Boeren, S. Vaessen, J. Vervoort, R. Pieters, Type 2 diabetes-related proteins derived from an in vitro model of inflamed fat tissue. Arch. Biochem. Biophys. 644, 81–92 (2018)
T.J. Lin, S.S. Yang, K.F. Hua, Y.L. Tsai, S.H. Lin, S.M. Ka, SPAK plays a pathogenic role in IgA nephropathy through the activation of NF-κB/MAPKs signaling pathway. Free Radic. Biol. Med. 99, 214–224 (2016)
H.S. Fernandes, T.C.S. Silva, P.A. Fernandes, M.J. Ramos, N.M. Cerqueira, Amino acid deprivation using enzymes as a targeted therapy for cancer and viral infections. Expert Opin. Ther. Pat. 27, 283–297 (2017)
G.M. Fischer, G.Y.N. Vashisht, J.L. McQuade, W. Peng, R.J. DeBerardinis, M.A. Davies, Metabolic strategies of melanoma cells: mechanisms, interactions with the tumor microenvironment, and therapeutic implications. Pigment Cell Melanoma Res 31, 11–30 (2018)
J. F. Trott, V. J. Hwang, T. Ishimaru, K. Chmiel, X. Zhou, K. Shim, B. Stewart, M. R. Mahjoub, K. Y. Jen, D. Barupal, X. Li, R. H. Weiss, Arginine reprogramming in ADPKD results in arginine-dependent cystogenesis. Am. J. Physiol. Renal Physiol. 315, F1855–F1868 (2018)
Y.S. Shan, H.P. Hsu, M.D. Lai, M.C. Yen, W.C. Chen, J.H. Fang, T.Y. Weng, Y.L. Chen, Argininosuccinate synthetase 1 suppression and arginine restriction inhibit cell migration in gastric cancer cell lines. Sci. Rep. 5, 9783 (2015)
D.L. Plubell, P.A. Wilmarth, Y. Zhao, A.M. Fenton, J. Minnier, A.P. Reddy, J. Klimek, X. Yang, L.L. David, N. Pamir, Extended multiplexing of tandem mass tags (TMT) labeling reveals age and high fat diet specific proteome changes in mouse epididymal adipose tissue. Mol. Cell Proteom. 16, 873–890 (2017)
W. C. Mu, E. VanHoosier, C. M. Elks, R. W. Grant, Long-term effects of dietary protein and branched-chain amino acids on metabolism and inflammation in mice. Nutrients 10(7), 918 (2018)
C.Y. Tsai, H.C. Chi, L.M. Chi, H.Y. Yang, M.M. Tsai, K.F. Lee, H.W. Huang, L.F. Chou, A.J. Cheng, C.W. Yang, C.S. Wang, K.H. Lin, Argininosuccinate synthetase 1 contributes to gastric cancer invasion and progression by modulating autophagy. FASEB J. 32(5), 2601–2614 (2018). https://doi.org/10.1096/fj.201700094R
M.M. Ragy, S.M. Ahmed, Protective effects of either C-peptide or l-arginine on pancreatic β-cell function, proliferation, and oxidative stress in streptozotocin-induced diabetic rats. J. Cell Physiol. 234(7), 11500–11510 (2019)
Funding
This study was funded by Science and technology innovation joint fund project Fujian Province (grant numbers 2016Y9102), Grants from National Natural Science Foundation of China (grant numbers 81500632 and 81870572), Natural Science Foundation of Fujian Province (grant number 2019J01455 and 2015J01453), Fujian Province Higher Education Institute New Century Research Project (grant number 2018B049) and Medical Innovation Fund Project of Fujian Province (grant numbers 2018-CX-23).
Author contributions
S.Y., Z.J. and L. C. wrote manuscript; X.S. conducted the design of the study and reviewed/edited the drafts, and is guarantor; B.F. researched data and edited the drafts; L.L. and Y.Y. contributed to discussion.Y.L. supplied the western blot experiment of islet and participated in modification of the manuscript. X.S. and L.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Yan, S., Jiang, Z., Cheng, L. et al. TLR4 knockout can improve dysfunction of β-cell by rebalancing proteomics disorders in pancreas of obese rats. Endocrine 67, 67–79 (2020). https://doi.org/10.1007/s12020-019-02106-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02106-5