Abstract
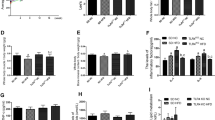
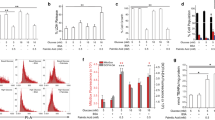
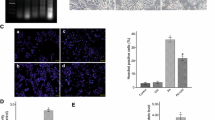
The Roux-en-Y gastric bypass (RYGB) is a one-of-a-kind treatment among contemporary bariatric surgical procedures, and its therapeutic effects for type 2 diabetes mellitus (T2DM) are satisfactory. The present study performed isobaric tags for relative and absolute quantification (iTRAQ) combined with liquid chromatography-tandem mass spectrometry (LC–MS/MS) analysis identifying different proteomics between T2DM rats with or without Roux-en-Y gastric bypass (RYGB) surgery, and GTP binding elongation factor GUF1 (Guf1) was first found to be significantly upregulated in rats from the T2DM plus RYGB group. In the cellular lipotoxicity model induced by palmitic acid stimulation of rat pancreatic beta cell line, INS-1, palmitic acid treatment inhibited cell viability, suppressed GSIS, promoted lipid droplet formation, promoted cell apoptosis, and induced mitochondrial membrane potential loss. The effects of palmitic acid on INS-1 cells mentioned above could be partially eliminated by Guf1 overexpression but aggravated by Guf1 knockdown. Last, under palmitic acid treatment, Guf1 overexpression promotes the PI3K/Akt and NF-κB signaling but inhibits the AMPK activation. Guf1 is upregulated in T2DM rats who received RYGB, and Guf1 overexpression improves cell mitochondrial functions, increases cell proliferation, inhibits cell apoptosis, and promotes cell functions in palmitic acid-treated β cells.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Polonsky KS (2012) The past 200 years in diabetes. N Engl J Med 367(14):1332–1340
Kahn SE, Hull RL, Utzschneider KM (2006) Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444(7121):840–846
Ye R, Onodera T, Scherer PE (2019) Lipotoxicity and beta cell maintenance in obesity and type 2 diabetes. J Endocr Soc 3(3):617–631
Yazici D, Sezer H (2017) Insulin resistance, obesity and lipotoxicity. Adv Exp Med Biol 960:277–304
Obeid F et al (2005) Open Roux-en-Y gastric bypass in 925 patients without mortality. Am J Surg 189(3):352–356
Hubens G et al (2008) Roux-en-Y gastric bypass procedure performed with the da Vinci robot system: is it worth it? Surg Endosc 22(7):1690–1696
Zhang S et al (2017) Increased beta-Cell mass in obese rats after gastric bypass: a potential mechanism for improving glycemic control. Med Sci Monit 23:2151–2158
Lindqvist A et al (2014) Gastric bypass improves beta-cell function and increases beta-cell mass in a porcine model. Diabetes 63(5):1665–1671
Camastra S et al (2011) Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia 54(8):2093–2102
Nguyen KT et al (2015) Preserved insulin secretory capacity and weight loss are the predominant predictors of glycemic control in patients with type 2 diabetes randomized to Roux-en-Y gastric bypass. Diabetes 64(9):3104–3110
Gass M, Beglinger C, Peterli R (2011) Metabolic surgery-principles and current concepts. Langenbecks Arch Surg 396(7):949–972
Dixon JB et al (2011) Bariatric surgery: an IDF statement for obese type 2 diabetes. Diabet Med 28(6):628–642
Halu A et al (2018) Context-enriched interactome powered by proteomics helps the identification of novel regulators of macrophage activation. Elife 7:e37059
Lee LH et al (2019) XINA: a workflow for the integration of multiplexed proteomics kinetics data with network analysis. J Proteome Res 18(2):775–781
Singh SA et al (2014) Co-regulation proteomics reveals substrates and mechanisms of APC/C-dependent degradation. EMBO J 33(4):385–399
Bauerschmitt H, Funes S, Herrmann JM (2008) The membrane-bound GTPase Guf1 promotes mitochondrial protein synthesis under suboptimal conditions. J Biol Chem 283(25):17139–17146
Gatmaitan P et al (2010) Pancreatic islet isolation after gastric bypass in a rat model: technique and initial results for a promising research tool. Surg Obes Relat Dis 6(5):532–537
Qi Y et al (2016) Quantitative proteomics reveals FLNC as a potential progression marker for the development of hepatocellular carcinoma. Oncotarget 7(42):68242–68252
Kinner A et al (2008) Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res 36(17):5678–5694
Nagelkerke, A. and P.N. Span, Staining against phospho-H2AX (γ-H2AX) as a marker for DNA damage and genomic instability in cancer tissues and cells. Adv Exp Med Biol, 2016. 899.
Rohlenova K, Neuzil J, Rohlena J (2016) The role of Her2 and other oncogenes of the PI3K/AKT pathway in mitochondria. Biol Chem 397(7):607–615
Herzig S, Shaw RJ (2018) AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol 19(2):121–135
Tattoli I et al (2008) NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kappaB and JNK pathways by inducing reactive oxygen species production. EMBO Rep 9(3):293–300
Seth RB et al (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122(5):669–682
Luo P et al (2016) Metabolomics study of Roux-en-Y gastric bypass surgery (RYGB) to treat Type 2 diabetes patients based on ultraperformance liquid chromatography-mass spectrometry. J Proteome Res 15(4):1288–1299
Arima N et al (2020) Multiorgan systems study reveals Igfbp7 as a suppressor of gluconeogenesis after gastric bypass surgery. J Proteome Res 19(1):129–143
Zhou X et al (2020) Quantitative proteomic analysis of porcine intestinal epithelial cells infected with porcine deltacoronavirus using iTRAQ-coupled LC-MS/MS. J Proteome Res 19(11):4470–4485
Zhao Z et al (2019) iTRAQ-based comparative proteomic analysis of cells infected with Eimeria tenella sporozoites. Parasite (Paris, France) 26:7
Xia H et al (2021) Exploration of identifying novel serum biomarkers for malignant mesothelioma using iTRAQ combined with 2D-LC-MS/MS. Environ Res 193:110467
Sun X et al (2019) Differential protein expression profiling by iTRAQ-2D-LC-MS/MS of rats treated with oxaliplatin. J Cell Biochem 120(10):18128–18141
Leloup C et al (2009) Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 58(3):673–681
Las G, Oliveira MF, Shirihai OS (2020) Emerging roles of beta-cell mitochondria in type-2-diabetes. Mol Aspects Med 71:100843
Coen PM et al (2015) Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes 64(11):3737–3750
Verbeek J et al (2015) Roux-en-y gastric bypass attenuates hepatic mitochondrial dysfunction in mice with non-alcoholic steatohepatitis. Gut 64(4):673–683
Sacks J et al (2018) Effect of Roux-en-Y gastric bypass on liver mitochondrial dynamics in a rat model of obesity. Physiol Rep 6(4):e13600
Jahansouz C et al (2015) Roux-en-Y gastric bypass acutely decreases protein carbonylation and increases expression of mitochondrial biogenesis genes in subcutaneous Adipose tissue. Obes Surg 25(12):2376–2385
Hudish LI, Reusch JE, Sussel L (2019) beta Cell dysfunction during progression of metabolic syndrome to type 2 diabetes. J Clin Invest 129(10):4001–4008
Christensen AA, Gannon M (2019) The beta cell in type 2 diabetes. Curr Diab Rep 19(9):81
Liao Z et al (2019) Polysaccharide from okra (Abelmoschus esculentus (L.) Moench) improves antioxidant capacity via PI3K/AKT pathways and Nrf2 translocation in a type 2 diabetes model. Molecules 24(10):1906
Huang W et al (2021) Metabolomics analysis on obesity-related obstructive sleep apnea after weight loss management: a preliminary study. Front Endocrinol 12:761547
Funding
This study was funded by the Wisdom Accumulation and Talent Cultivation Project of the Third Xiangya Hospital of Central South University (No. YX202106).
Author information
Authors and Affiliations
Contributions
Weizheng Li and Pengzhou Li conceptualized and designed the experiments. Pengzhou Li drafted the article. Weizheng Li revised the article critically for important intellectual content. Pengzhou Li, Song Dai, and Xiang Gao contributed to cell and animal experiments. Song Dai and Xiang Gao contributed to the analysis and manuscript preparation. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Approval of the research protocol
N/A.
Informed consent
N/A.
Approval date of registry and the registration no. of the study/trial
N/A.
Animal studies
The animal experiments were approved by the Ethics Committee of Central South University (approval no. 2019–S383).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Guf1 was upregulated in pancreatic tissues in T2DM rats with RYGB surgery.
• Guf1 improves palmitic acid-induced dysfunctions of rats’ islet β cells.
• Guf1 improves palmitic acid-induced mitochondrial dysfunction and apoptosis.
• Guf1 promotes the PI3K/Akt and NF-κB signaling but inhibits the AMPK activation.
Supplementary Information
Additional file 1: Fig. S1.
Determination of Mrps16 and Guf1 expression. (A) Mrps16 and Guf1 expression in pancreatic tissues from T2DM and T2DM+RYGB rats. (B) Mrps16 and Guf1 expression in INS-1 cells treated with or without PA.
Additional file 2: Fig. S2.
Effects of lentivirus of overexpressing Guf1 or knockdown Guf1 on INS-1 cells viability. INS-1 cells were non-transduced or transduced with lentivirus overexpressing Guf1 (lv-Guf1) or lentivirus knockdown Guf1 (sh1-Guf1 or sh2-Guf1) and then examined for cell viability by MTT assay.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, P., Dai, S., Gao, X. et al. Guf1 overexpression improves pancreatic β cell functions in type 2 diabetes mellitus rats with Roux-en-Y gastric bypass (RYGB) surgery. J Physiol Biochem 79, 569–582 (2023). https://doi.org/10.1007/s13105-023-00952-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-023-00952-6