Abstract
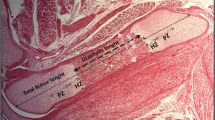
We aimed to investigate the effect of maternal exposure to NaF on mandibular bone microarchitecture and phosphocalcic plasma parameters of the offspring. For this purpose, 10-, 15-, and 21-day-old pups (n = 6–8 per group) from two groups of mothers, control and NaF 50mg/L treated dams, were used. Plasma calcium (Ca) and phosphorus (P) levels and alkaline phosphatase activity (ALP) were measured. Fluoride concentration (F−) in bone and in stomach content was measured using potentiometry after isothermal distillation. Morphometric, histological, and histomorphometric analyses of the jaw bones were performed. Plasma Ca and P levels and ALP activity increased in 10-day and decreased in 21-day-old pups from NaF-treated mothers. Fluoride concentration in stomach content samples of 15- and 21-day-old nursing pups from mothers exposed to NaF in their drinking water was higher compared to that observed in control dam offspring. Mandibular F− content was higher in 21-day-old pups born to F−-exposed dams compared to those observed in age-matched control pups. Mandibular area increased in 21-day-old pups born to treated mothers as compared to controls. Mandibular bone volume BV/TV (%) was higher in offspring from NaF-exposed dams than in controls at all the studied times. The increase in bone volume after exposure to F− was concomitant with the increase in trabecular thickness and the decrease in trabecular separation. Altogether, our results showed that exposure to NaF during gestation and lactation increased mandibular area and bone volume of pups, with concomitant changes in phosphocalcic parameters associated with the bone modeling process.






Similar content being viewed by others
References
DenBesten P, Li W (2011) Chronic fluoride toxicity: dental fluorosis. Monogr Oral Sci 22:81–96. https://doi.org/10.1159/000327028
Perumal E, Paul V, Govindarajan V, Panneerselvam L (2013) A brief review on experimental fluorosis. Toxicol Lett 223(2):236–251. https://doi.org/10.1016/j.toxlet.2013.09.005
Ohmi K, Nakagaki H, Tsuboi S, Okumura A, Sugiyama T, Thuy TT, Robinson C (2005) The effect of fluoridation and its discontinuation on fluoride profiles in the alveolar bone of rat. Calcif Tissue Int 77(4):226–232. https://doi.org/10.1007/s00223-004-1304-5
Buzalaf MA, Whitford GM (2011) Fluoride metabolism. In: Buzalaf M (ed). Fluoride and the oral environment. Monogr Oral Sci Karger 22:20–36. https://doi.org/10.1159/000325107
Everett ET (2011) Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J Dent Res 90(5):552–560. https://doi.org/10.1177/0022034510384626
Qu H, Wei M (2006) The effect of fluoride contents in fluoridated hydroxyapatite on osteoblast behavior. Acta Biomater Res 2(1):113–119. https://doi.org/10.1016/j.actbio.2005.09.003
Pan L, Shi X, Liu S, Guo X, Zhao M, Cai R, Sun G (2014) Fluoride promotes osteoblastic differentiation through canonical Wnt/β-catenin signaling pathway. Toxicol Lett 225(1):34–42. https://doi.org/10.1016/j.toxlet.2013.11.029
Qu WJ, Zhong DB, Wu PF, Wang JF, Han B (2008) Sodium fluoride modulates caprine osteoblast proliferation and differentiation. J Bone Miner Metab 26(4):328–234. https://doi.org/10.1007/s00774-007-0832-2
Nanci A (2008) Ten Cate’s oral histology: development, structure, and function, 8th edn. Mosby Elsevier, St Louis
Sanz-Salvador L, García-Pérez MA, Tarín JJ, Cano A (2015) Bone metabolic changes during pregnancy: a period of vulnerability to osteoporosis and fracture. Eur J Endocrinol 172:53–65
Brambilla E, Belluomo G, Malerba A, Buscaglia M, Strohmenger L (1994) Oral administration of fluoride in pregnant women, and the relation between concentration in maternal plasma and in amniotic fluid. Arch Oral Biol 39:991–994
Gurumurthy SM, Shruti M, Sapna V, Aparna VB, Pragna R (2011) Transplacental transport of fluoride, calcium and magnesium. Natl J Int Res Med 2:51–55
Messer HH, Armstrong WD, Singer L (1974) Effect of maternal fluoride intake on preweaning bone fluoride concentrations in mice. J Dent Res 53(1):145. https://doi.org/10.1177/00220345740530011501
Speirs RL (1986) The relationship between fluoride concentrations in serum and in mineralized tissues in the rat. Arch Oral Biol 31(6):373–381. https://doi.org/10.1016/0003-9969(86)90160-3
Narayanaswamy M, Piler MB (2010) Effect of maternal exposure of fluoride on biometals and oxidative stress parameters in developing CNS of rat. Biol Trace Elem Res 133(1):71–82. https://doi.org/10.1007/s12011-009-8413-y
Rigalli A, Alloatti R, Puche RC (1999) Measurement of total and diffusible serum fluoride. J Clin Lab Anal 13:151–157
Eratalay YK, Simmons DJ, El-Mofty SK, Rosenberc GD, Nelson W, Haus E, Halberg F (1981) Bone growth in the rat mandible following every-day or alternate-day methylprednisolone treatment schedules. Arch Oral Biol 26(10):769–777. https://doi.org/10.1016/0003-9969(81)90172-2
Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM (2013) Standardized nomenclature, symbols, and units for bone Histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry nomenclature committee. J Bone Miner Res 28(1):2–17. https://doi.org/10.1002/jbmr.1805
Grynpas MD, Chachra D, Limeback H (2000) The action of fluoride on bone. In: Henderson JE, Goltzman D (eds) The osteoporosis primer. Cambridge University Press, Cambridge, pp 318–330
Drinkard CR, Deaton TG, Bawden JW (1985) Enamel fluoride in nursing rats with mothers drinking water with high fluoride concentrations. J Dent Res 64(6):877–880. https://doi.org/10.1177/00220345850640060301
Saiani RA, Porto IM, Marcantonio Junior E, Cury JA, De Sousa FB, Gerlach RF (2009) Morphological characterization of rat incisor fluorotic lesions. Arch Oral Biol 54(11):1008–1015. https://doi.org/10.1016/j.archoralbio.2009.08.009
Centeno VA, Fontanetti PA, Interlandi V, Ponce RH, Gallará RV (2015) Fluoride alters connexin expression in rat incisor pulp. Arch Oral Biol 60(2):313–319. https://doi.org/10.1016/j.archoralbio.2014.11.003
Dunapice A, Brizendine E, Zhang W, Wilson M, Miller L, Warrick B, Stookey G (1995) Effect of aging on animal response to chronic fluoride exposure. J Dent Res 74:358–368
Buzalaf MA, Moraes CM, Olympio KP, Pessan JP, Grizzo LT, Silva TL, Magalhães AC, Oliveira RC, Groisman S, Ramires I (2013) Seven years of external control of fluoride levels in the public water supply in Bauru, São Paulo, Brazil. J Appl Oral Sci 21(1):92–98. https://doi.org/10.1590/1678-7757201302196
Whitford GM (1999) Fluoride metabolism and excretion in children. J Public Health Dent 59(4):224–228. https://doi.org/10.1111/j.1752-7325.1999.tb03273.x
Schour I, Massler M (1949) The teeth. In: Farris EJ, Griffith JQ (eds) The rat in laboratory investigation, J. B. Lippincott Company, Philadelphia, pp 104–160
Sener Y, Tosun G, Kahvecioglu F, Gökalp A, Koç H (2007) Fluoride levels of human plasma and breast milk. Eur J Dent 1:21–24
Seifert M, Nelke KH, Noczyńska A, Łysenko L, Kubacka M, Gerber H (2015) Bone markers in craniofacial bone deformations and dysplasias. Postepy Hig Med Dosw 28:1176–1181
Chandrajith R, Dissanayake CB, Ariyarathna T, Herath HM, Padmasiri JP (2011) Dose-dependent Na and ca in fluoride-rich drinking water--another major cause of chronic renal failure in tropical arid regions. Sci Total Environ 409:671–675
Zhou Z, Wang H, Zheng B, Han Z, Chen Y, Ma Y (2017) A rat experimental study of the relationship between fluoride exposure and sensitive biomarkers. Biol Trace Elem Res 180:100–109
Huang H, Ba Y, Wu JJ, Li EW, Ma JG, Li SH et al (2007) Effects of the contents of Ca2 and Mg2 in drinking water on serum alkaline phosphatase in fluoride exposed population. J Zhengzhou Univ (Med Sci) 42:55–57
Song Y, Tan H, Liu KJ, Zhang Y, Liu Y, Lu C, Yu D, Tu J, Cui C (2011) Effect of fluoride exposure on bone metabolism indicators ALP, BALP and BGP. Environ Health Prev Med 16(3):158–163. https://doi.org/10.1007/s12199-010-0181-y
Wise GE, Yao S, Henk W (2007) Bone formation as a potential motive force of tooth eruption in the rat molar. Clin Anat 20:632–639
Wise GE, King GJ (2008) Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res 87(5):414–434. https://doi.org/10.1177/154405910808700509
Wise GE (2009) Cellular and molecular basis of tooth eruption. Orthod Craniofacial Res 12(2):67–73. https://doi.org/10.1111/j.1601-6343.2009.01439.x
Acknowledgements
The authors are very grateful to Germán Tirao for his technical assistance and to Alfredo Rigalli for his generous and dedicated collaboration. This work was funded by SECyT-Universidad Nacional de Córdoba (UNC) No 202/2016 and FO-UNC: 242/2014 (Viviana Centeno), CICyT-Universidad Nacional de La Rioja (Raquel Gallará).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal protocol was approved by the Bioethics Committee of the School of Medicine of the National University of Córdoba (Argentina) and is in keeping with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No 8023, revised 1978).
Conflict of Interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Rights and permissions
About this article
Cite this article
Interlandi, V., Fontanetti, P.A., Ponce, R.H. et al. Chronic Exposure to Fluoride During Gestation and Lactation Increases Mandibular Bone Volume of Suckling Rats. Biol Trace Elem Res 185, 395–403 (2018). https://doi.org/10.1007/s12011-018-1258-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1258-5