Abstract
Patulin is a mycotoxin whose presence in apple-derived products and fruit juices is legally regulated, being its maximum limits established in the legislation of multiple countries. However, the management of contaminated batches is still an issue for producers. This investigation aims to evaluate ultraviolet light (254 nm, UV-C254nm) irradiation to find solutions that can be applied at different stages of the apple juice production chain. In this regard, 8.8 (UV-1) and 35.1 (UV-2) kJ m−2 treatments inactivated spores of Penicillium expansum CMP-1 on the surface of apples. Although the same treatments applied to wounded apples (either before the infection or after the infection, immediately or when the lesion had appeared) did not show any effect on the growth rate of P. expansum during storage (up to 14 days, at 4 or 25 °C), they reduced patulin content per lesion size in apples treated after the infection had occurred (patulin decreased from 2.24 (control) to 0.65 µg kg−1 cm−2 (UV-2 treated apples)). Additionally, the treatment of juice with patulin with ultraviolet light up to 450.6 kJ m−2 resulted in more than 98 % reduction of patulin. Degradation products of patulin after UV-C254nm treatments were tentatively identified by HPLC–MS, and toxicity and biological activities were assessed in silico, and results indicated that such products did not pose an increased risk when compared to patulin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patulin is a secondary metabolite (polyketide lactone, 4-hydroxy-4H-furo [3,2-c]pyran-2(6H)-one) produced by molds. It is a common mycotoxin contaminant in food that can result in acute and subacute toxicity and chronic symptoms. After its toxic effect was identified, it was categorized under group 3 (non-classifiable as a carcinogen) by the International Agency for Research on Cancer (IARC, 2018). However, other proven harmful impacts were found on health (tested in mice), including immunotoxicity, hepatotoxicity, gastrointestinal, and neurological problems (Vidal et al., 2019). Patulin in food has been identified in a variety of agricultural crops (tomatoes, peppers), various fruits (apples, pears, grapes), and cereals (rice, ground cereals) (Ngolong Ngea et al., 2020). Amongst these, the high-water content and high concentration of sugars in fruit make such products a suitable media for patulin-producing mold (Penicillium, Aspergillus, and Byssochlamys species). For this reason, legislation establishes maximum limits of patulin, focusing on apple products, for being the most common sources of this mycotoxin (Mahato et al., 2021). In this regard, the European Union Commission Regulation establishes maximum patulin levels of 50 µg kg−1 in fruit juices and nectars, 25 µg kg−1 for applesauce and other solid products from apple, and 10 µg kg−1 if such products are for short-age children (CR(EC)No1881/2006). The US Food and Drug Administration (FDA) and the China Food and Drug Administration (CFDA) have also regulated maximum patulin levels of 50 µg kg−1 in fruit juices (FDA, 2005; CFDA, 2017).
Despite these regulations, patulin presence in food commodities across the world is still an issue for food industries (especially for fruit and juice processors), and products exceeding the established limits are still encountered (Sajid et al., 2019). For this, methods to minimize patulin concentrations in such products are needed and being developed. In the case of fruit processing, some critical steps condition the patulin load in the final product. First, the entrance to the process of fruit batches contains contaminated pieces with mold conidia and/or with already decayed pieces by patulin-producing molds. Second, during the storage time prior to juice or derivate products processing (from weeks up to 3 months), the fruit is subjected to conditions favorable for mold growth and for patulin production. Once mycotoxins are formed, it is difficult to manage their amounts, as they are typically stable during storage and insensitive to most physical and chemical treatments (Bullerman & Bianchini, 2007). In fact, it is reported that patulin is quite stable at high temperatures (105–125 °C) in aqueous solutions at pH 3.5–5.5 (fruits and juices) (Lovett & Peeler, 1973). For this reason, the main patulin prevention strategies are focused on reducing the incidence and growth of the causative molds or affecting their ability to produce the mycotoxin. In this regard, and as it has been extensively reviewed in Sajid et al. (2019), biological strategies are being investigated, and those include microbial control (using yeast, bacteria, and fungi), the use of antifungal biomolecules, or their combination. Also, the use of ClO2 treatments has been evaluated, but patulin degradation in apple juice has been slightly affected (Ran et al., 2019).
Another alternative that has been under study in the last years is the application of ultraviolet light that involves the irradiation of the product with the electromagnetic spectrum ranging from 100 to 400 nm. This strategy has raised interest amongst fruit processors due to the lack of toxic by-products generated, no production of off-odors and off-tastes affecting the treated products, and the lower energy requirements when compared to other processes applied in this industry (Riganakos et al., 2017). In fact, Vignali et al. (2022) have recently reported that the energy consumption of this technology is just the 6–8 % of the energy consumption of conventional heat treatments, ohmic treatments, microwaves, high hydrostatic pressure, or pulsed electric fields.
In the fruit industry, ultraviolet light has proved to be able to inactivate spores from various fungal species (Begum et al., 2009). In fact, the wavelength that ranges from 200 to 280 nm (UV-C) is the most germicidal. This wavelength range coincides with the maximum absorption of DNA (260 nm) and, therefore, can block its replication and compromise the cell function (Guerrero-Beltrán & Barbosa-Cánovas, 2004). Amongst the species that can produce patulin, Penicillium expansum is the main producer of patulin in apples, pears, and their derived products (McKinley & Carlton, 1991). In addition to the fact that most of the P. expansum strains are patulin producers (Garcia et al., 2011; Tannous et al., 2018), this specie causes 80–90 % of the rot decay in apples (Viñas et al., 1995). For this reason, controlling the infection caused by this mold can result in patulin control and minimize fruit loss with its consequent reduction of economic costs.
Applications of UV-C light irradiation to control P. expansum have shown that UV-C dose to reduce 2 log conidia g−1 of P. expansum population in apple surface was 1.03 kJ m−2 (Syamaladevi et al., 2015). Other studies required between 2.5 and 10 kJ m−2 to reduce 2.8 ± 0.4 log units of the spores of P. expansum in apple peel (Rios de Souza et al., 2020). Moreover, the application of low doses of UV-C light could elicit hormesis or a series of stress responses from the plant that include the production of anti-fungal compounds and ripening delay (Shama & Alderson, 2005). In this regard, irradiation (7.5 kJ m−2) of “Red Delicious” apples 24 to 96 h before the inoculation of P. expansum resulted in a reduction of the area under disease caused by this mold during storage. Moreover, if P. expansum DNA is damaged during ultraviolet light irradiation, it may limit its capacity to synthesize patulin, as the biosynthesis pathways of this mycotoxin involve 15 genes in the same cluster that encode some proteins, such as PatE and PatH, and eight essential enzymes (Li et al., 2019).
Treatments using UV-C are also being applied to apple juice and cider with the purpose to degrade patulin. For instance, according to Tikekar et al. (2014), 50 % of patulin (initial content 1,000 µg kg−1) was reduced after irradiation of apple juice with 30 kJ m−2. In another study, higher reductions of 72 % of patulin content (initial content 1,000 µg kg−1) were achieved with the application of lower UV-C doses (0.9 kJ m−2) (Assatarakul et al., 2012). Although there are several papers commenting on the reduction of patulin, differences may be encountered and can be attributed to juice absorbance, to sample treatment procedures, or to equipment configuration. Despite this, patulin has been proven to be reduced with this technology, but no research has been found that surveyed degradation products from patulin under UV-C application. In fact, compared to patulin, the toxicology of other patulin-related metabolites (mainly produced by biocontrol microorganisms in patulin degradation) is less investigated. Three patulin degradation products have been widely reported, which are desoxypatulinic acid, two isomers of ascladiol (E- and Z-ascladiol), and hydroascladiol (Ianiri et al., 2017; Shao et al., 2012). Other studies have reported that UV-C did not significantly increase the cytotoxicity and mutagenicity of aflatoxins B1 and M1 in milk, but no studies focusing on patulin degradation by UV-C have been found (Kurup et al., 2022). For this, patulin UV-C degradation products and their toxicity should be assessed as reactions could result in lower molecular weight substances that could enter blood stream more easily and have dissimilar effects on human body.
This paper aims to investigate different strategies using UV-C light at a wavelength of 254 nm (UV-C254nm) application to minimize patulin concentrations on apple products. For this, the effects of UV-C254nm on P. expansum (main producing agent) were studied for (i) spore inactivation, (ii) growth prevention and lesion development in apples, and (iii) patulin production ability. Moreover, the application of UV-C light on apple juice was performed in order to (iv) study degradation kinetics of this mycotoxin in the matrix and specially (v) tentatively identify the degradation compounds of patulin and (vi) elucidate the main toxicological aspects of such compounds.
Materials and Methods
Penicillium expansum Propagation and Apple Inoculation
Penicillium expansum strain CMP-1 was acquired from Colección Española de Cultivos Tipo, CECT-20906 (Valencia, Spain). Prior to the experimentation, the strain was stored at –80 °C in agar cubes suspended in water with 20 % (v/v) glycerol. P. expansum was propagated onto potato dextrose agar (PDA, 200 mL boiled potato extract, 20 g dextrose, 20 g agar, and 800 mL water) at 25 °C for 7 days for conidiation. Inoculum was prepared by conidia suspension on sterile water with 0.5 % (w/v) Tween-80, counted on a Thoma Haemocytometer Counting Chamber and adjusted to the desired concentration (5 × 104 conidia mL−1) by dilution.
Apples cv. “Golden Delicious” with no post-harvest treatments were kindly provided by a local producer. Fruits were cleaned with tap water and stored at 4 °C until use within the next 7 days. Apples were divided in batches, used for the different “case study” scenarios, and distributed in individual alveoli in plastic boxes (two boxes per treatment and scenario). A schematic explanation of the experimental design is shown in Fig. 1. For surface inoculation, an area of 2.54 cm2 was marked on three spots on peel (total area 7.62 cm2) in the side of the apple (Colás-Medà et al., 2021). For wound inoculation, a wound of 2 mm wide × 1 mm depth was cut with a sterile scalpel (Syamaladevi et al., 2014). This was done in the side of the apple because fruit wounds may occur in any part of the fruits, and it facilitated the lesion size measurements. Then 20 µL of the conidia suspension was spot inoculated to achieve 1 × 103 conidia on each mark or wound, respectively. Samples were dry for 1 h at room temperature next to Bunsen burners to prevent ambient contaminations. Surface and inoculated apples were positioned horizontally (wounded side upwards) with the help of plastic alveoli.
Preparation of Juice Containing Patulin
Juice Preparation
To prepare apple juice for the evaluation of patulin degradation by UV-C254nm, wound inoculated apples with a P. expansum CMP-1 conidia suspension at 5 × 104 conidia mL−1 as explained in the “Penicillium expansum Propagation and Apple Inoculation” section were stored at 25 ± 1 °C for 9 days. Then, juice was prepared using a cold-press blender ZM1501 (AMZCHEF, USA) and filtered with lab paper through a kitasato assisted with vacuum to eliminate suspended particles. Patulin content in the elaborated juice was determined by HPLC–DAD following the procedure described in the “HPLC–DAD Quantification of Patulin” section and adjusted to 1,000 µg kg−1 (a concentration 20-fold higher than the regulated limit for this kind of products, to be able to observe reductions in highly contaminated juices) apple juice prepared in the same way from healthy apples. With the purpose to verify that fate of patulin under UV-C254nm in spiked samples and in the elaborated juice is comparable, juice was spiked (spiked juice) with patulin (≥ 98.0 %, HPLC grade, Sigma-Aldrich). For this, apple concentrate provided by Dallan, S.A. Moleva with patulin levels below the limit of detection of the method (HPLC–DAD Quantification of Patulin Section) was reconstituted with sterile tap water to meet the criteria regarding the quality characteristics of the juice established in legislation (“Commission Directive 2001/112/EC relating to fruit juices and certain similar products intended for human consumption”, CD2001/112/EC). An aliquot of patulin, which was stored in ethyl-acetate (EA) at –20 °C, was evaporated, resuspended in distilled water, and added to the juice to reach a desired concentration of 1,000 µg kg−1.
In order to evaluate degradation products of patulin under UV-C254nm treatment, a model juice solution was prepared with a concentration of patulin of 50,000 µg kg−1 and 1,000 µg kg−1. It consisted of distilled water with 5 g L−1 malic acid (VWR International, Prolabo), 115 g L−1 sucrose (D-sucrose anhydrous, Fisher Scientific), and the adequate volume of reconstituted patulin in distilled water to reach the desired concentration. This was done to prevent matrix interferences for any possible degradation products. Once degradation products peaks were tentatively identified (Identification of Patulin Degradation Products by HPLC–MS section), they were determined in lower patulin levels (9,000 and 1,000 µg kg−1) in the elaborated apple juice samples.
Juice Quality Evaluation
The quality of the juice (both elaborated from contaminated apples and spiked with patulin) was evaluated according to the “Commission Directive 2001/112/EC relating to fruit juices and certain similar products intended for human consumption” (CD2001/112/EC) to check if it complied with the criteria regarding quality characteristics legislated thereby. The pH value was recorded using a pH 50 VioLab benchtop pH meter equipped with a 201 T Electrode, and TSS values were determined as described in the “Apple Quality Parameters Evaluation” section. Juice density (g L−1) was calculated using Eq. 1 with a 25 mL pycnometer to determine the mass of the solutions:
where dj is the density of the juice (in g L−1), m0 is the mass of the empty pycnometer (g), mw is the mass of the pycnometer with water (g), mj is the mass of the pycnometer with juice, and dw is the density of water (g L−1) at the working temperature.
The juice transmittance (% T) was calculated from the measured absorbance (A) at 254 nm using a spectrophotometer (UV-1600PC, VWR International, USA) and using the following Eq. 2:
Effect of UV-C254nm Light on P. expansum Conidia, Its Ability to Infect Apples, and Its Capacity to Produce Patulin
Treatments with UV-C254nm Light for Apple-Matrix Experiments
For UV-C254nm light irradiation, a module for surface disinfection eos® UV OF 5050 (Peschl Ultraviolet GmbH, Germany) was used. This equipment consists on a stainless steel horizontal chamber (1,155 × 272 × 133 mm, L × W × H) with an irradiation area of 518 × 206 mm. In the upper part, 20 lamps emitting light at 254 nm (UV-C254nm) to a total of 760 W m−2 (according to manufacturers’ indications) are set. This setup was used for its convenient size to work with whole fruits. Apples placed in plastic alveoli were distributed within the irradiation area (total of 12 could be treated each time), with the inoculated surface facing the lamps, at a distance of 5 cm from it. The UV-C254nm intensity during treatments was monitored by a UV-sensor Easy HW (Peschl Ultraviolet, Germany) radiometer that was placed in the same position than the samples. Three treatments were proposed: control treatment (CT), consisting of non-irradiated samples and irradiated samples at two different UV-C254nm doses: 8.8 ± 0.1 kJ m−2 (UV-1) and 35.1 ± 0.4 kJ m−2 (UV-2).
Irradiation dose was calculated using Eq. 3 proposed by Kowalski (2009):
Inactivation of P. expansum Conidia on Apple Surface
This scenario represents the case in which P. expansum conidia are contaminating fruit surfaces without a wound. In this condition, the spores will not infect the fruit and cause visible growth or decay, but they can enter the fruit chain and represent the source of cross-contamination of wounded fruits in the packing house, where the mold will be able to grow. Each of the treatments (CT, UV-1, and UV-2, performed as explained in the “Treatments with UV-C254nm Light for Apple-Matrix Experiments” section) was applied to 6 apples with 3 marked areas on each (n = 6). Immediately after the treatments, viable conidia were quantified. For this, the three marked areas per repetition were sampled with a sterile scalpel and introduced in a sterile filter bag (BagPage®, Interscience Bag System, France) and homogenized on peptone buffered water (BPW, Biokar) by means of a paddle blender (IUL, Spain) for 1.5 min (250 impact min−1). Then, 1 mL per duplicate was plated onto Dichloran Rose-Bengal Chloramphenicol (DRBC) agar plates and incubated at 25 ± 1 °C for 5 days. Characteristic P. expansum colonies were counted, and results were expressed as log conidia cm−2. Detection limit was 0.1 log conidia cm−2.
Inactivation of P. expansum in Wounded Apples: Effect in Lesion Size and Patulin Content
In this section, three different scenarios were proposed. For each treatment (CT, UV-1, and UV-2, performed as explained in the “Treatments with UV-C254nm Light for Apple-Matrix Experiments” section), 6 repetitions were evaluated, consisting of 3 apples per repetition.
-
(i)
Pre-infection
In this scenario, apples with non-inoculated wounds were treated, and wounds were inoculated 24 h later to mimic a posterior contamination. During the 24-h time, apples were stored at 4.3 ± 0.4 °C. This intended to simulate the possible recontamination of apples with remaining conidia after the irradiation with UV-C light. Moreover, the pretreatment of apples was proposed to study any possible hormetic effect, affecting further host-pathogen interactions.
-
(ii)
Post-infection
This scenario mimics the case in which P. expansum conidia have reached apple wounds but have not infected the fruit yet. Apples with inoculated wounds were treated 24 h after the inoculation, during which apples were stored at 4.3 ± 0.4 °C.
-
(iii)
With lesion
This scenario simulates the irradiation of apples to which P. expansum has reached the wound and caused a lesion (1.0 cm) to the apple. Treatments were applied to wounded and inoculated apples after 7 days storage at 4.3 ± 0.4 °C plus 4 days storage at 24.9 ± 1.3 °C, to mimic chamber storage and posterior manipulation, until lesions reached 1.0 cm Ø.
After the treatments, plastic boxes containing apples were stored at room temperature (24.9 ± 1.3 °C) for 14 days or until diameter lesion reached approximately 3 cm. The incidence of mold growth and subsequent decay was calculated in percentage (proportion of decayed apples to total inoculated apples). The severity of lesions (mean of two perpendicular diameters, cm) at each measurement time (day) was plotted, and growth rate (cm day−1) for each sample was calculated from the slope by linear regression.
At the end of each storage period, sample preparation for patulin quantification was performed as explained in the “Sample Preparation” section.
Apple Quality Parameters Evaluation
Initial quality and quality after the treatments (immediately and after 24 h of cold storage at 4.3 ± 0.4 °C) were determined in 9 apples (3 repetitions, 3 apples each) that were left uninoculated for this purpose. Moreover, non-wounded and non-inoculated samples (n = 3, 3 apples each) were treated equally to the inoculated samples and subjected simultaneously to the same conditions explained for wound-inoculated samples explained in the “Inactivation of P. expansum in Wounded Apples: Effect in Lesion Size and Patulin Content” section.
Quality evaluation included firmness, pH, total soluble solids (TSS), and titratable acidity (TA). Firmness was evaluated with a pocket penetrometer FT 327 (Facchini SRL, Italia) with a 113-mm rod that was introduced 8 mm in 2 opposed points of each apple. Firmness was expressed as kg cm−2. The pH values were recorded using a penetration probe T205 (Testo, Germany) on two opposed points of each apple. Then, juice of the 3 apples per repetition was prepared using a cold-press blender ZM1501 (AMZCHEF, USA). The TSS values were determined by duplicate measurements using a handheld refractometer PAL-1 (ATAGO, Japan) and expressed as %. For TA measures, 10 mL of juice were diluted with 10 mL distilled water and titrated with 0.1 M NaOH (VWR, USA) until pH 8.2 was reached. A conversion factor of 0.67 was used to express results as malic acid, in g L−1.
Effect of UV-C254nm Light on Patulin Content of Apple Juice
Treatments Using UV-C254nm in Apple Juice
The treatments of apple juice (elaborated juice and spiked juice) with patulin to evaluate patulin degradation kinetics were performed on a laboratory-scale UV-C254nm light equipment, previously described in Nicolau-Lapeña et al. (2022). It consisted of a chamber (618 × 277 × 20 mm, L × W × H) equipped with three monochromatic UV-C lamps (254 nm, 30 W). Apple juice was poured onto 12-well plates distributed on the tray (Falcon, USA) containing 1.2 mL of sample per well (4 mm depth), which was not stirred. Irradiation doses in the surface of the juice ranged from 43.3 ± 3.3 to 450.6 ± 8.1 kJ m−2, dose at which no patulin was detected. The UV-C254nm intensity during treatments was monitored by a UV-sensor Easy HW (Peschl Ultraviolet, Germany) radiometer that was placed in the same position than the samples. One milliliter of juice was collected per triplicate at each evaluated dose. This experiment was replicated twice for elaborated juice and twice for spiked juice with patulin (n = 6).
To determine the degradation products of patulin under UV-C254nm, model juice solution and elaborated juice (prepared as explained in the “Preparation of Juice Containing Patulin” section) were treated with four selected irradiation doses: 76.0 ± 1.0, 152.1 ± 1.2, 304.8 ± 1.0, and 453.8 ± 2.5 kJ m−2. The control sample consisted of the corresponding juice that remained untreated.
Patulin Degradation Kinetics
Patulin degradation was modeled to determine degradation kinetics. It was adjusted to a zero-, first-, and second-order rate models and assessed using the relevant integrated rate equations, as follows (Eqs. 4, 5, and 6):
where [P] is the patulin concentration (µg kg−1); [P]0 indicates initial concentration; k is the rate constant [(µg kg−1 kJ−1 m2) for zero-order model, (kJ−1 m2) for the first-order model, and (µg−1 kg kJ−1 m2) for the second-order model]; and i is the UV-C254nm irradiation dose (kJ m−2).
Patulin Quantification
Sample Preparation
For patulin quantification in apple samples, 6 repetitions (consisting on 3 apples each) per treatment and scenario were evaluated. Preparation of samples was performed according to Morales et al. (2007) with small modifications. Briefly, the lesions were sampled with a 5 cm Ø sharp core-borer until core axis was reached. The 1 cm extra per side (lesions of 3 cm Ø) was sampled in case of patulin diffusion to the non-damaged tissue. The three-lesion pool was homogenized with distilled water (proportion 3:1) by means of a blender (Moulinex Turbomix 350 W, France). Then, 200 µL of pectinase (Sigma, 3,800 U mL−1) were added, to the puree and incubated during 1 h at 40 °C, to prevent emulsions during extraction. At this point, samples were frozen at –80 °C until further manipulation. In the day of the extraction, samples were tempered and centrifuged at 10,000 × g for 5 min, to separate the liquid from the pulp. An aliquot of 3 mL of the puree liquid was tubed in duplicate for further extraction.
For patulin quantification in apple juice, 3 mL of apple juice were determined per repetition (n = 6).
Patulin Extraction and Concentration
Patulin from the liquid matrices (purée or the different evaluated juices) was extracted following the official method AOAC 995.10 (Brause et al., 1996). Briefly, the 3-mL aliquots were extracted (per duplicate) three times with 6 mL EA, cleaned up by 1.5 % (w/v) Na2CO3 (Panreac Quimica), and filtered through Na2SO4 (Rectapur) to remove water impurities. Then, an aliquot of 9 mL EA was evaporated and resuspended to 1 mL of Milli-Q water pH 4.0 (adjusted with acetic acid 6.1 M). Extracts were filtered through 0.22 µm Ø prior to HPLC injection.
HPLC–DAD Quantification of Patulin
Patulin was quantified following the guides of the official method AOAC 995.10 (Brause et al., 1996). For this, a HPLC Infinity 1260 (Agilent Technologies, USA) coupled to a DAD system (II G7117C, Agilent Technologies, USA) was employed. An isocratic solvent system was used (0.8 % tetrahydrofuran; HPLC grade, Fisher-Chemical), and the flow rate was fixed at 1 mL min−1. Patulin from the injected 50 µL was separated on a Gemini® column (C18, 5 µm, 118 Å, 150 × 4.5 mm) (Phenomenex, USA) and detected at 276 nm. The identification of patulin was performed by comparing the retention time with that of a previously obtained standard, and quantification was done by interpolation in a calibration curve prepared in the adequate concentration range for each experiment. Detection limit of the method was 5 µg kg−1. Patulin in apple was expressed as µg kg−1 cm−1, where cm−1 expressed the size (diameter) of the lesion, in order to compare patulin contents between similar size lesions.
Study of Patulin Degradation Products Under UV-C254nm Light
Identification of Patulin Degradation Products by HPLC–MS
After the treatment of the model juice solution spiked with patulin (prepared as detailed in the “Juice Preparation” section) and the evaluation of the degradation degree of patulin at the selected treatment doses evaluated by injection in the HPLC–DAD system as described in the “HPLC–DAD Quantification of Patulin” section, the degraded samples and a non-degraded reference-sample were injected in a capillary liquid chromatographic system coupled to a mass spectrometer (cLC-MS). The cLC-MS system was Agilent system Mod. 1100 Series equipped with a binary capillary pump and coupled to a 6120 simple quadrupole analyzer (Agilent, California, USA). Then, 10 μL were injected by using an external loop positioned into a Rheodyne® injection valve. A Phenomenex Luna C18 (2) column (150 mm; 0.30 mm; 3 μm particle size; 100 Å pore size) was used. The mobile phases were A (water-0.1 % formic acid) and B (acetonitrile), operated in isocratic mode (92 % A and 8 % B) at a flow of 8 μL min−1. The electrospray ionization (ESI) source was operated in positive mode, and the ESI parameters were nebulizer gas (N2) (350 °C; 35 psi, and 12.0 L min−1) and the capillary voltage at 3.5 kV. The data were acquired in scan mode (from 50 to 250 m/z) and/or in SIM mode (m/z = 155; 195 and 111). The tentative identification of the degraded products of patulin was carried out by studying the scan chromatograms of degraded synthetic samples (on the base of molecular ions observed) and by elucidating their possible structure on base of the reactivity of patulin and bibliography related. Samples were injected at different dilution levels.
In Silico Toxicity and Biological Activity Evaluation of Patulin Degradation Products
The evaluation of the possible toxicity and biological activities of the possible structures of the degraded compounds with respect to patulin and to other degradation compounds that are typically formed from patulin degradation and two web tools were used. The chemical structures were generated by using ChemSketch (ADC/labs; Ontario, Canada). Biological activities and Lipinski’s rule were calculated by using the Molinspiration software version 2020.08 (www.molinspiration.com). The biological activities evaluated were the G-protein coupled receptor (GPCR) ligand, ion channel modulator, kinase inhibitor, nuclear receptor ligand, protease inhibitor, and enzyme inhibitor, where higher scores values indicate higher activity. Moreover, to evaluate the permeability across the cell membrane, the Lipinski’s rule of five was employed, which establishes that for a good permeability across the cell membrane, the compound must meet these criteria: (a) octanol/water partition coefficient lower than 5 (log p < 5), (b) molecular weight lower than 500 Da, (c) number of hydrogen bond donors (nitrogen and/or oxygen) less than 5, and (d) number of hydrogen bond acceptors (nitrogen and/or oxygen) less than 10. To be orally active, it must have no more than one violation of this rule (Lipinski et al., 2001). In addition, other molecular parameters also were obtained such as molecular polar surface area, number of rotatable bonds, and molecular volume. To evaluate the toxicity of the degradation compounds (mutagenesis, tumorigenesis, irritant, and effects on the reproductive system), the Osiris explorer software (www.organic-chemistry.org/prog/peo/) was used. The results are expressed in colors, being black a high risk, followed by grey, and white being low risk or drug-conform behavior.
Statistical Analysis
Data were checked for significant differences between treatments (for P. expansum and patulin experiments, n = 6; for quality parameter evaluation, n = 3) by applying analysis of variance test (ANOVA). The criterion for statistical significance was p < 0.05. When significant differences were observed, Tukey’s Honest Significant Difference (HSD) of the means was applied using JMP 15.1 (SAS Institute Inc., Cary, USA). Linear regressions were calculated using Microsoft Excel (Microsoft Corporation, USA).
Results
Inactivation of P. expansum Conidia on Apple Surface by UV-C254nm
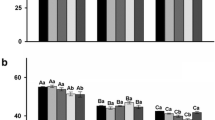
P. expansum CMP-1 conidia spot inoculated onto apple peel surface initial population was 2.95 ± 0.04 log conidia cm−2 (Fig. 2). After UV-1 treatment (8.8 kJ m−2), viable conidia had decreased by 2.7 log units, and after UV-2 treatment (35.1 kJ m−2), growth of colonies from viable conidia were not detected, achieving an inactivation level of more than 99.9 %.
P. expansum Infectivity of Wounded Apples and Patulin Production After UV-C254nm Treatments
The effect that UV-C254nm had on the ability of P. expansum CMP-1 to infect (cause lesion) to wounded apples and to produce patulin was evaluated in different scenarios. Incidence was not affected by any of the treatments in the proposed scenarios, where all the apples developed lesions (100 % incidence, data not shown). The growth rate of P. expansum CMP-1 in the apples was evaluated by measuring the severity of the lesion during the storage time, and it was not significantly (p < 0.05) affected by UV-C254nm treatments in any of the scenarios (Fig. 3). In the first scenario, in which the UV-C254nm were applied 24 h before the infection, the growth rate of the lesion ranged from 0.36 ± 0.14 to 0.42 ± 0.07 cm day−1. In the scenario in which UV-C254nm was applied to wounded and infected apples, growth rate ranged from 0.48 ± 0.17 to 0.51 ± 0.12 cm day−1. In apples with 1.0 cm lesion, the growth rate ranged from 0.37 ± 0.17 to 0.54 ± 0.19, regardless of the treatment. Patulin content of the apples at the end of the storage period or until halos have reached 3.0 cm Ø ranged from 1,805.2 ± 186.9 (post-infection, UV-2) to 6,783.8 ± 508.2 (pre-infection, UV-2) µg kg−1, depending on the treatment. Patulin content was expressed in relation to lesion size to relate patulin production with P. expansum CMP-1 infection. Patulin content per lesion size in the first scenario (pre-infection) was not significantly (p < 0.05) affected by UV-C254nm treatment and averaged 2,247.6 ± 124.9 µg kg−1. Contrarily, in the case of the second and third scenarios (treatments post-infection and treatments in apples with lesion), UV-C254nm significantly (p < 0.05) affected patulin content per lesion size. In the case of post-infection treatments, the decrease in these values was correlated to the increase in irradiation dose: 2,238.5 ± 247.18, 1,488.7 ± 239.1 (30.5 % decrease compared to the control), and 664.0 ± 78.0 µg kg−1 cm−1 (70.3 % decrease compared to the control), for CT, UV-1 (8.8 kJ m−2), and UV-2 (35.1 kJ m−2) treatments, respectively. In the case of the UV-C254nm treatments applied to apples with 1.0 cm lesion, patulin content per lesion size significantly (p < 0.05) decreased from 2,517.5 ± 99.1 µg kg−1 cm−1 (CT) to an average of 1,384.0 ± 59.7 µg kg−1 cm−1(UV-1 and UV-2, 45.0 % decrease compared to control).
Effect of UV-C254nm Irradiation on Selected Apple Quality Parameters
Apple quality parameters were evaluated immediately after and after 24 h of irradiation treatments, to see if any immediate effect on apples occurred due to irradiation (Table 1). The initial quality of the apples used in this study is represented by CT treatment at 0 h, as it represents the non-irradiated samples at the beginning of the experiment. Initial values of pH, firmness, TSS, and TA were 3.8 ± 0.0, 7.5 ± 0.2 kg cm−2, 13.6 ± 0.4 %, and 3.3 ± 0.1 malic acid in g L−1. No significant differences (p < 0.05) were found in these values immediately after the UV254nm treatments. After 24 h of storage at 4 °C, irradiated samples followed similar evolution to non-irradiated samples, as no significant differences were observed between such values after this period.
Moreover, the quality parameters of apples were investigated after the storage period for each of the scenarios, and results were compared amongst treatments within the same scenario (Table 2), to see if irradiation with UV254nm had an impact on apples that could correlate with P. expansum virulence or its ability to produce patulin. In general, pH and firmness slightly decreased, TSS increased, and TA values were maintained or decreased. Although some significant differences (p < 0.05) were detected between treatments in some cases (pH in pre-infection scenario, firmness in pre- and post-infection scenario, and TSS in post-infection scenario), no general pattern relating UV-C254nm dose with observed changes could be elucidated.
Patulin Degradation in Apple Juice by UV-C254nm Irradiation
Juice used in this experiment (elaborated juice and spiked juice) followed the required quality criteria legislated by EU regulations: pH of 3.60 ± 0.04, TSS of 12.0 ± 0.3 %, density 1,045.0 ± 1.0 g L−1. Moreover, transmittance was < 0.01 %. Both juices, either elaborated from contaminated apples or spiked with patulin were subjected to UV-C254nm irradiation for patulin degradation. Degradation kinetic models and goodness of fit expressed by R2 are shown in Table 3. The fit of data from both samples properly adjusted to first-rate kinetics model (R2 = 0.9542 and 0.9821 for elaborated or spiked juice, respectively) and revealed that the rate constant was similar between them (− 0.0081 and − 0.0082 kJ−1 m2, for elaborated or spiked juice, respectively) (Fig. 4A). For the similarity in the behavior, degradation of patulin under UV-C254nm in juice is presented joint (expressed in %) regardless of the juice matrix (Fig. 4B). Degradation model for patulin according to the “Patulin Degradation Kinetics” section was then [P] = 1,000 e −0.0082i. For instance, at a starting point of 1,000 µg kg−1 of patulin in the juice, the remaining percentages after 88.7, 224.7, and 450.6 kJ m−2 were 58.3, 20.8, and 1.4 %.
Viable conidia (expressed in log conidia cm−2) of P. expansum CMP-1 spot inoculated onto apple surface after UV-C254nm light irradiation treatments. Treatments include control treatment (CT), UV-C254nm irradiation at 8.8 kJ m−2 (UV-1) and UV-C254nm irradiation at 35.1 kJ m−2 (UV-2). Bars represent the mean (n = 6) and the standard error for each parameter. Different letters mean statistically significant differences between treatments (p < 0.05), according to Tukey’s HSD test
Patulin Degradation Compounds Generated After UV-C254nm Irradiation and In Silico Biological and Toxicological Evaluation
The identification of the patulin degradation compounds after treatments with UV-C254nm irradiation in real apple juice may present a strong analytical difficulty, mainly caused by the challenge of obtaining clean samples adequate for the analysis by LC–MS. For this reason, experiments using a model juice solution imitating the main composition of apple juice were performed. Similar destruction percentages were observed for model juice solution and elaborated juice. Patulin was detected for m/z = 155 (molecular weight of patulin 154) at 5.5 min, decreasing in the treated samples as shown in Table 4. The deep study of the chromatograms obtained in scan mode (m/z from 50 to 250) has allowed observing two peaks with m/z 111 and 195 (both of them around 3.8 min) in UV-C254nm-treated synthetic samples that could be degradation products of patulin. Figure S1 (in supplementary material) presented the chromatograms (in SIM mode) for these two peaks for synthetic (A) and elaborated (B) juices. As can be seen in model juice solution samples, the peak of m/z = 111 showed a maximum at 304.8 ± 1.0 kJ m−2. In the case of the peak m/z = 195, the maximum was observed at 152.1 ± 1.2 kJ m−2. For elaborated juice samples, in both cases, other compounds coeluted close to this time; however, a trend can be observed for the high irradiation doses that could be related to the formation of these degraded products.
In consideration of the structure of patulin, and based on several possible mechanisms described in the bibliography for other compounds and/or treatments (such as aflatoxin UV-C treated and patulin ozone treated), UV-C light could induce several interactions in the molecule: (a) hydration or hydrogenation of double bounds to form simple bounds; (b) reduction of alcohol groups to C = O; and (c) a hypothesized bond breakdown C-O and C-(C = O) of rings to lose CO2 (Diao et al., 2019; Patras et al., 2017). Considering, for the m/z ions found in the cLC-MS analysis, different structures have been proposed for each (Fig. 5). Thus, for m/z = 111, which corresponds to M + 1, therefore, the molecular mass would be 110 g mol−1. In this case, the most likely reaction is the loss of CO2 (breakdown of C-O and C-(C = O)) and the reduction of alcohol to ketone, being at least three possibilities for this structure (Fig. 5b-c-d). For the ion m/z = 195, it can be considered a sodium adduct (M + 23) from the degraded compound, so its molar mass should be 172 g mol−1. It could be caused by the hydration of one of the double bonds of patulin (Fig. 5e–f).
Patulin content, normalized to lesion size (white bars, left axis, µg kg−1 cm−1) and growth rate of lesion (black diamonds, right axis, cm day−1) in apples after storage time for the different presented scenarios: 24 h prior to the infection with P. expansum CMP-1 (pre-infection), 24 h after the infection (post-infection), and when P. expansum growth had reached 1.0 cm lesion (with lesion). Treatments include control treatment (CT), UV-C254nm irradiation at 8.8 kJ m−2 (UV-1), and UV-C254nm irradiation at 35.1 kJ m−2 (UV-2). Values are the mean (n = 6) and the standard error for each parameter. Within the same scenario, different letters mean statistically significant differences between treatments (p < 0.05), according to Tukey’s HSD test
a Degradation of patulin in elaborated juice (black diamonds) and in spiked juice (gray dots) expressed as ln [P]/[P]0 in front of irradiation dose (kJ m−2) for first-order kinetic modeling. Values are the mean (n = 6) ant the standard error. Linear regression slope values represent k values for each matrix (kinetic constant, 1/kJ m−2). b Remaining patulin content in (%) for each irradiation dose (kJ m−2). Bars represent the mean (n = 6) and the standard error. The model of remaining patulin ([P]) depending on the irradiation dose (i, kJ m−2) is shown in the graphic
Chemical structures of patulin a and the proposed degradation products of patulin under UVC254nm light (continuous arrow), estimated structures for ion m/z = 111, molecular mass 110 g mol−1 b, c, d and estimated structures for ion 'sm/z = 195, molecular mass 172 g mol.−1 e, f, and the most reported degradation products in literature, being ascladiol g, hydroascladiol h, and deoxypatulinic acid i
To evaluate the possible activity and toxicity of the tentative structures proposed as patulin degradation products via UV-C254nm light irradiation, two in silico tests were performed. The information obtained for the proposed degradation products was compared to that obtained for patulin and for the other products reported in the literature obtained from degradation mainly via microorganisms (ascladiol, hydroascladiol, and deoxypatulinic acid, whose structures are shown in Fig. 5g-h-i). This information on the estimation of the biological and toxicological activities of the mentioned compounds is shown in Table 5. As can be seen, it seems that biological activities of estimated structures of 110 g mol−1 have lower activities when compared to patulin (e.g., GPCR ligand activity ranging from −3.69 to −3.13 compared to −1.65 of patulin or enzyme inhibitor activity of compound 110 ranging from −2.61 to −2.53 compared to that of patulin, 0.35). However, estimated structures for 172 g mol−1 seem to have, overall, more activity compared to that of patulin. When compared to the other degradation compounds reported in the literature, the three estimated structures for compound 110 have lower biological activity, while structures proposed for compound 172 have similar (higher or lower in some cases) activities. On the other hand, taking into account that none of these compounds showed any violation of Lipinski’s rules, and also considering the values of the molecular polar surface area (all of them < 140 Å2) and the number of rotatable bonds (< 10), apparently, no differences in absorption can be predicted respect to the patulin (Lipinski et al., 2001; Veber et al., 2002).
Regarding the toxicity evaluation, patulin is highly mutagenic, tumorigenic, and has an effect on the reproductive system, while it has no risk of being irritant. Divergences in toxicities have been found for the proposed degraded products. For instance, there is a high risk for compounds 110-A and 110-C to be irritants and for compound 110-C to be tumorigenic. However, mutagenesis is only categorized as medium risk for compound 110-B, and tumorigenesis is indicated only for compounds 110-B and 110-C. Moreover, none of the degradation structures seem to affect the reproductive system. On the other hand, structures of molecular weight of 172 showed no risk in any parameter.
Discussion
Inactivation of P. expansum CMP-1 Conidia, Growth control of P. expansum CMP-1 in Apples, and Patulin Production Minimization by Means of UV-C254nm
To evaluate the direct effect of ultraviolet light in P. expansum CMP-1 conidia, the inoculated surface of apples was irradiated with two different doses. Inactivation of viable conidia by UV-C254nmhas been already reported for different mold genres and species. For instance, Aspergillus niger, Aspergillus flavus, and Penicillium corylophilum on agar surface were reduced by ca. 80–99 % after exposure to UV-C light (1.16 kJ m−2)(Begum et al., 2009). Similarly, Valero et al. (2007) proved the complete inactivation of different species (A. niger, Cladosporium herbarum, Alternaria alternata, and Penicillium janthinellum) after 0.015–0.225 kJ m−2. In that study, the susceptibility of Penicillium genre to UV-C light was higher due to the few protective mechanisms against UV-C irradiation it has: produces single-celled conidia, which are slightly pigmented. In fact, it is suggested that melanin-like compounds may help microorganisms protect themselves from irradiation (Dadachova & Casadevall, 2008). Such compounds and other pigments may block the penetration of UV-C light, by shielding the irradiation and scattering it, preventing its incidence to the DNA, and avoiding or minimizing damage and formation of pyrimidine nucleotides interfering with RNA transcription and DNA replication (Seltsam & Müller, 2011). In this regard, the surface morphology of the fruit may also influence the efficacy of UV-C irradiation on fungi conidia. Amongst the fruits studied in Syamaladevi et al. (2015) (apples, cherries, strawberries, and raspberries), apples were more susceptible to P. expansum inactivation due to low hydrophobicity and low roughness of the surface, decreasing the required dose to reduce 2 log CFU g−1 of P. expansum population (1.03 kJ m−2) when compared to that of raspberries (1.61 kJ m−2). As highlighted in Gayán et al. (2014), effects on spores could be subjected to prior sublethal stresses and post-recovery conditions after UV-C treatments; however, this issue has not been addressed in the present paper, as we have focused on studying other critical factors such as the virulence and high lesion size producing P. expansum strain (Torres et al., 2003).
While pre-harvest infections occur through the calyx or along the stem, post-harvest molds (as P. expansum) can infect fruit in all areas of the surface during the harvest and post-harvest steps (such as storage, packaging, transportation, and processing operations) (Tournas, 2005) if being wounded. Penicillium spp. requires a wound to infect the host, and unlike other fungi, it does not produce appressoria (being then unable to penetrate the intact epidermis of fruits and vegetables) (Errampalli, 2014). This suggests that irradiation of apples via portable devices in the harvest process, or in the first steps in the post-harvest manipulation, is key to minimizing the incidence of P. expansum.
Conversely, when conidia were inoculated onto 2 mm wounds, UV-C254nm treatments did not significantly influence the incidence or the severity of the lesions when compared to the control. This could be explained via the incomplete inactivation of all the viable conidia due to shadowing effect provoked by the wound or by the stacked conidia or due to the internalization of the microorganism in the fruit tissue (Manzocco et al., 2011). In this sense, Gündüz and Pazir (2013) compared the efficacy of UV-C254nm irradiation (15.84 kJ m−2) on P. digitatum and P. italicum artificially inoculated in oranges via three methods: spot, wound, and piercing. While in spot inoculation (surface) a 100 % inactivation occurred, only 43.5 or 8.6 % inactivation was obtained in wounded or pierced fruits. The reason to inoculate P. expansum CMP-1 on a piercing (called wound in this paper) in the apples was then to simulate a worst-case scenario in which contamination can occur and can persist even after UV-C irradiation. Being the elimination of viable conidia from the surfaces more feasible than it is when conidia have penetrated the wound, it could be applied as a preventive treatment for P. expansum in apples. As it is in post-harvest when numerous wounds may occur either mechanically or by chilling injuries making fruit more prone to fungi spoilage to the softer tissue, a UV-C pretreatment results in a reduction of cross-contamination or prevention of conidia to penetrate the wounds (Saleh & Goktepe, 2019). Once in the wound, and due to the limited penetration of UV-C into the tissue, great survival is possible for P. expansum in the interstitial spaces and wounds of fruit tissue (Syamaladevi et al., 2013).
Despite UV-C254nm has proven effective in inactivating viable conidia, the presence of a wound has challenged the control of the severity of the lesions, demonstrating that residual conidia hidden from UV-C254nm are still capable to infect the fruit. However, this study has been unable to demonstrate that UV-C254nm light can affect growth ability or speed of P. expansum CMP-1. Other studies show that differences between inoculum levels can affect the incidence value and the lag phase and growth rate of the lesion (Baert et al., 2008; Morales et al., 2008). For instance, Baert et al. (2008) showed that inoculation with P. expansum at 2 × 104 conidia mL−1 resulted in the 90 % of apples infected, while this value decreased when inoculum size was lower. Also, lag phase increased when inoculum size decreased. In the case of the present study, either lag phase was not affected by a decrease in viable conidia or conidia could not be decreased in a significant extent to affect infectivity and growth rate.
Moreover, and in contrast to earlier findings, no evidence of hormesis was detected. A relationship between sub-lethal UV-C irradiation doses and inhibition of incidence and severity of lesions of various molds on fruit has been reported in the literature. For instance, Yamaga and Nakamura (2019) found that irradiation of mandarins with 6 kJ m−2 at 24 h before the inoculation reduced Penicillium italicum mycelium and sporulation areas and also reduced the incidence of naturally occurring blue and green mold on the fruits. In apples, UV-C irradiation at 96 h before the inoculation was effective in reducing the diameter lesions of P. expansum disease (de Capdeville et al., 2002). An initial objective of the project was to assess possible hormetic effects in apples caused by UV-C254nm at 8.8 or 35.1 kJ m−2. It was hypothesized that some changes could be elicited in the apple in response to stress, and for this reason, the first scenario in which apples were irradiated 24 h prior to the inoculation was prepared. However, neither the physicochemical characterization of the apples nor the incidence and growth rate of P. expansum CMP-1 supported this idea. In fact, physicochemical parameters were mostly unaffected by UV-C254nm treatment, neither immediately or after 24 h nor after storage periods of the different scenarios. Despite some lower pH and firmness values (pre-infection CT and post-infection UV-2) were observed, physicochemical characteristics of apples in the present study ranged within the reported values in the literature (Calu et al., 2009). Moreover, as reported by several authors, UV-C treatments may not significantly affect the quality parameters of strawberries (17.2 W cm−2, 5 min, by Nicolau-Lapeña et al. (2020)), pears (3.6 kJ m−2, by Syamaladevi et al. (2014)), or caquis (3 kJ m−2, by Khademi et al., 2013).
The patulin accumulation observed in this study (ranging between ca. 660 and 2,500 µg kg−1 cm−1) is higher than that reported by Morales et al. (2008) but similar to other studies by Baert et al. (2007) and Reddy et al. (2010). The most interesting finding of this part of the investigation was that patulin production decreased in treatments where UV-C254nm light was applied after the inoculation (post-infection and with lesion scenarios). The observed decrease of patulin content ranged between 28.9 (UV-1 in comparison to CT in scenario post-infection) and 70.8 % (UV-2 in comparison to CT in the same scenario), averaging 40 % in the scenario in which apples were irradiated with a lesion. This lack of relationship between virulence of P. expansum (similar growth rates and incidence) and patulin production (decrease when UV-C254nm irradiated) has previously been observed by Morales et al. (2008). However, the insights behind this have already been elucidated genetically. Genes that play crucial roles in the biosynthesis of patulin production (PePatL, PePatK, and brlA) are not related to the virulence of P. expansum (Li et al., 2015) and not related to conidiation (Zetina-Serrano et al., 2020). Despite reducing patulin accumulation, UV-C treatments were not able to fully inactivate patulin production. In fact, according to the Food and Drug Administration Compliance Policy Guide concerning patulin (FDA, 2002), at such levels of patulin, if one rotten apple (containing more than 10 µg kg−1) is used with 200 sound apples to make juice, the resulting patulin level in the juice could exceed the established limits for patulin content in such products. Overall, the proposed UV-C254nm treatment can be applied with the purpose to reduce viable conidia on the surface of apples and prevent posterior contamination of wounds, decreasing the spoilage by fungi, that causes the 5–20 % of the total fruit loss (even when fungicides are used) (Cappellini & Ceponis, 1984). Further processes must be done to decrease patulin content in apple products, and for this reason, juice UV-C254nm irradiation was proposed next.
Patulin Degradation in Apple Juice by Means of UV-C254nm. Kinetics and Degradation Products
At the light that complete reduction of the risk of patulin presence on apple derivative products once P. expansum has reached the wound is challenging, juice (elaborated from contaminated apples or spiked) with patulin was treated with UV-C254nm light to evaluate patulin degradation in it. The presence of contaminated apples in the industrial processes can occur through inadvertent or negligent inclusions (Salomao et al., 2008), resulting in contaminated juice. For this reason, effective technologies are required for patulin reduction in processed products. Ultraviolet light is a clean technology adequate for this purpose, due to the absence of toxic by-products generated during the treatment, supposedly no production of off-tastes and off-odors of the treated products, and the lower requirement of energy when compared to other processing technologies (Riganakos et al., 2017). In this way, Caminiti et al. (2012) treated apple juice with UV-C and described that it did not affect pH, TSS, or phenolic content but decreased non-enzymatic browning and antioxidant activity (11 % decrease) when irradiation was higher than 265.5 kJ m−2. However, at lower doses, the color change was unnoticeable by a consumer panel. For the maintenance of the juice properties and the already reported capacity of UV-C to reduce patulin in apple juices, juice prepared with contaminated apples (elaborated juice) and juice in which patulin was added (spiked juice) to 1,000 µg kg−1 were subjected to UV-C254nm treatments. It must be noted that the irradiation dose was measured by a radiometer, so the dose expressed in this manuscript corresponds to that received by the surface of the juice. More precise and accurate methods should be used in further studies to determine the received dose by, for instance, the use of actinometry. This measure indicates the remaining energy that is not absorbed or scattered by the fluid, which is therefore available for microbial inactivation or patulin degradation (Guerrero et al., 2021; Koutchma et al., 2016).
Degradation of patulin was modelized, and the comparison of three kinetic models revealed that degradation was better adjusted to a first-order model. A first-order is a reaction that proceeds at a rate that depends linearly on one reactant concentration, in this case, patulin. Zero- and second-order models showed lower linear correlation coefficients, indicating a poorer adjustment to the data. Adjustment to a first-order kinetic model was in accordance with Assatarakul et al. (2012) and Zhu et al. (2014). In the first study, for instance, rate constant was − 0.0294 mJ−1 cm2 for apple juice and 0.0053 mJ−1 cm2 for cider, and in the second case, rate constant was − 0.0273 mJ-1 cm2. Differences in rate constants between the present and the literature studies can be attributed to the dissimilar setups of the experiment (flow, distance of the lamps) and to the intrinsic characteristics of the juice (turbidity, transmittance) (Fenoglio et al., 2020). Additionally, other equipment configurations have been used in the literature. The equipment used for the experimental part in this paper uses mercury lamps in a horizontal disposition, where the product passes below the lamps. Other configurations have been reported in the literature such as low pressure or medium pressure mercury lamps, with pulsed or continuous light (Orlowska et al., 2013), and light-emitting diodes, that can be distributed along the processing containers and conveyors (Khan et al., 2022; Nassarawa et al., 2020).
No differences were observed in patulin reduction regarding juice matrix (elaborated from contaminated apples or juice spiked with patulin), meaning that regardless patulin was bonded or attached naturally to juice compounds, UV-C254nm light accessibility and effect could be fully achieved. At a starting point of 1,000 µg kg−1 (20 times the maximum limit established by legislation), a treatment of 404.7 kJ m−2 would be needed to achieve a 95 % of reduction and meet legislation criteria. Additionally, we have previously reported reductions of 2.5 to 4 log units of Escherichia coli, Salmonella enterica, and Listeria monocytogenes populations in apple juice after the application of UV-C254nm at ca. 35 kJ m−2 (Nicolau-Lapeña et al., 2022). Other authors have reported similar results against E. coli O157:H7 and L. monocytogenes (Woo et al., 2020), but those are focused on spinach rather than on apple products. The irradiation doses necessary for patulin inactivation reported in the present study (up to 450.6 kJ m−2) are 10 times higher than those needed for microbial inactivation, resulting in a safer product from both points of view: microbiological and mycotoxicological. However, so high levels of contamination are not normally occurring in commercialized juices, and the maximum levels are up to 350 µg kg−1 (Bracket & Marth, 1979), 309 µg kg−1 (Ware et al., 1974), 375 µg kg−1 (Gockmen & Açar, 1999), or 45 µg kg−1 (Leggott & Shephard, 2001). Based on the first-kinetic model, and taking into account these more realistic patulin initial concentrations, the necessary dose for commercial uses should be lower, and minimizing treatment time or lamp intensity could be possible when scaling up. In escalation processes, equipment design should be carefully assessed, as maximizing exposed surface to UV-C light is of high importance, as penetration for 90 % of absorption in apple juices is reported to be 0.67 mm (Koutchma, 2009). Other papers have reported different approaches to overcome penetration problems and facilitate escalation of the UV-C treatment. As some examples, there are Taylor-Couette’s UV-C producing units (Orlowska et al., 2014), vertical concentric tubes (Caminiti et al., 2012), and collimated beam apparatus (Tikekar et al., 2014). As highlighted in the introduction, the application of UV-C light is focused on the reduction of patulin and the production of degradation molecules, for there is a lack of information in this regard. The impact that UV-C has on other juice parameters such as pH, color, acidity, odor, or taste is marginal and can be consulted elsewhere (Barut Gök, 2021; Caminiti et al., 2012; La Cava & Sgroppo, 2019). The effect of UV-C treatments should also be assessed considering other parameters that can negatively influence the quality of the juice, such as the formation of off-flavors. The formation of 5-hydroxymethyfurfural is a parameter to consider as it can interfere with the quantification of patulin by HPLC, but it usually leads to an overestimation of the mycotoxin (Shephard & Leggott, 2000). Vitamin C in fortified juices must also be considered because its addition may cause poor reproducibility in patulin results, as they can react one with the other (Brause et al., 1996). Moreover, this study was conducted on apple juice, and it would be interesting to study also other factors such as other fruits or that have undergone a clarification process, when adapting and scaling the UV-C treatments to the industry.
The study of the degradation products of patulin formed after the treatments with UV-C254nm irradiation is key to elucidating whether the risk of the obtained product is lower or this patulin structure alteration has led to the formation of more toxic products, e.g., by the breakdown into lower molecular weight compounds that can be easily absorbed. To date, studies evaluating degradation compounds of patulin are focused on bio-degradation (mainly by yeasts) (Ngolong Ngea et al., 2020) and on glutathione reactions (Rodríguez-Bencomo et al., 2020). However, the investigations focusing on UV-C applications to degrade patulin do not go further and do not determine or identify the formed compounds thereof. In the present study, two possible degradation mechanisms, leading to five proposed structures, were identified. According to the obtained chromatograms, the compound with a mass of 110 g mol−1 and the compound with a mass of 172 g mol−1 have their maximums at 304.8 and 152.1 kJ m−2, respectively. This could be attributed to the further degradation of such compounds via UV-C254nm to form other structures. However, this behavior could not be observed clearly in juice since the complexity of the juice matrix would require a more complex analysis approach. Although it could be assumed that similar reactions would occur in elaborated juices (and at usual concentrations of patulin), more investigation in this regard is needed in real matrices, to identify these compounds and other products that may be formed and to evaluate the sequential degradations that can occur with higher treatments.
Regarding the toxicity of patulin, it is believed that it mainly interacts with sulfhydryl groups, for which it has a strong affinity, explaining the inhibition of many enzymes (Puel et al., 2010). Although only patulin levels present in some foodstuffs are regulated, and degradation via UV-C has proven to lower them, ideally, the degraded products should have less biological activity, limited oral absorption, and lower toxigenic risk when compared to the parental molecule (patulin). The in silico evaluation of the proposed structures has added some valuable information on the feasibility to use UV-C to degrade patulin. Although the Lipinski’s rules (and other related parameters) do not allow obtaining conclusions regarding the oral absorption of these compounds, it has been found that one of the degradation products (110 g mol−1) has less biological activity and, in general, less (or similar) toxicity than patulin, and the other compound (172 g mol−1) is less toxic than patulin. Indeed, when compared to other degradation compounds that have been reported in the literature and that are typical of bio-degradation processes, those obtained after UV-C254nm treatments have similar or lower biological activities and toxicity (except for that of 110 g mol−1 which can be tumorigenic). This indicates that UV-C254nm treatments are, at least, equally effective in reducing patulin toxicity in food products that bio-degradation processes using microorganisms may be. This represents an advantage in food products that can more easily treated by UV-C light or in processes where the use of microorganisms is not possible or not advisable. Despite this, to obtain more conclusive evidence, more studies should focus on degradation products’ toxicity of different juice matrices, considering the different structures that can be formed initially and with subsequent treatments.
Conclusions
This paper evaluates the application of UV-C254nm irradiation to different points of the apple juice production chain to minimize patulin content in the final product. From the outcomes of the study, two main conclusions have been obtained. In the first place, treatment of unwound apples with ultraviolet light for 8.8 kJ m−2 would be advisable to be applied in the first steps of the production chain, specifically before storage. The inability of P. expansum to produce decayed lesions in intact apples combined with the low efficacy of the treatment once it has infected a wound makes the surface treatment the best option to prevent cross-contamination of damaged apples and further mold growth, with its subsequent patulin production. In the second place, such measures, applied together with careful handling of the product, fail or are insufficient to prevent patulin limit-exceeding contents, adding a processing step in the juice production chain consisting of a UV-C254nm treatment. This treatment has proven effective to reduce more than 98 % of the patulin content even when it is 20 times higher than the regulated limits (25 µg kg−1). However, further studies should be performed using continuous-flow lab-scale reactors, bypasses, or in recirculation mode to optimize juice treatment. In them, the delivered dose measured with more accurate methods should also be taken into account. Moreover, studies carried out in identifying the possible toxicity of the degradation products generated thereof (tentatively identified) suggest that they may have similar or lower toxicity than patulin and other derived products generated by other degradation methods. The incorporation of ultraviolet treatment in juice-producing industries and its integration with other good handling and patulin control practices can reduce the economic losses by contaminated batches, and patulin exceeding legislation limits alerts may be minimized, offering the consumers safer products.
Data Availability
Data is available under reasonable demand to the corresponding author.
Abbreviations
- CT:
-
Control treatment
- TA:
-
Titratable acidity
- TSS:
-
Total soluble solids
- UV-C:
-
Ultraviolet C
- UV-C254nm :
-
Ultraviolet C light at 254 nm that was used in the experimental part of the manuscript
- UV-1:
-
Ultraviolet C treatment 1 (8.8 kJ m−2)
- UV-2:
-
Ultraviolet C treatment 2 (35.1 kJ m−2)
- k:
-
Kinetic constant
References
Assatarakul, K., Churey, J. J., Manns, D. C., & Worobo, R. W. (2012). Patulin reduction in apple juice from concentrate by UV radiation and comparison of kinetic degradation models between apple juice and apple cider. Journal of Food Protection, 75(4), 717–724. https://doi.org/10.4315/0362-028X.JFP-11-429
Baert, K., Devlieghere, F., Bo, L., Debevere, J., & De Meulenaer, B. (2008). The effect of inoculum size on the growth of Penicillium expansum in apples. Food Microbiology, 25(1), 212–217. https://doi.org/10.1016/j.fm.2007.06.002
Baert, K., Devlieghere, F., Flyps, H., Oosterlinck, M., Ahmed, M. M., Rajkovic, A., Verlinden, B., Nicolai, B., Debevere, J., & De Meulenaer, B. (2007). Influence of storage conditions of apples on growth and patulin production by Penicillium expansum. International Journal of Food MIcrobiology, 119(3), 170–181. https://doi.org/10.1016/j.ijfoodmicro.2007.07.061
Barut Gök, S. (2021). UV-C treatment of apple and grape juices by modified UV-C reactor based on Dean vortex technology: Microbial, physicochemical and sensorial parameters evaluation. Food and Bioprocess Technology, 14(6), 1055–1066. https://doi.org/10.1007/s11947-021-02624-z
Begum, M., Hocking, A. D., & Miskelly, D. (2009). Inactivation of food spoilage fungi by ultra violet (UVC) irradiation. International Journal of Food Microbiology, 129(1), 74–77. https://doi.org/10.1016/j.ijfoodmicro.2008.11.020
Bracket, R. E., & Marth, E. H. (1979). Patulin in apple juice from roadside stands in Wisconsin. Journal of Food Protection, 42(11), 862–863.
Brause, A. R., Trucksess, M. W., Thomas, F. S., & Page, W. S. (1996). Determination of patulin in apple juice by liquid chromatography: Collaborative study. Journal of AOAC International, 79, 451–455.
Bullerman, L. B., & Bianchini, A. (2007). Stability of mycotoxins during food processing. International Journal of Food Microbiology, 119(1–2), 140–146. https://doi.org/10.1016/j.ijfoodmicro.2007.07.035
Calu, M., Bonciu, C., & Tofan, I. (2009). Physico-chemical characteristics of apples stored in chilling and controlled atmosphere conditions. The Annals of the University Dunarea de Jos of Galati Fascicle VI – Food Technology, 34(October), 32–37.
Caminiti, I. M., Palgan, I., Muñoz, A., Noci, F., Whyte, P., Morgan, D. J., Cronin, D. A., & Lyng, J. G. (2012). The effect of ultraviolet light on microbial inactivation and quality attributes of apple juice. Food and Bioprocess Technology, 5(2), 680–686. https://doi.org/10.1007/s11947-010-0365-x
Cappellini, R., & Ceponis, M. (1984). Postharvest losses in fresh fruits and vegetables. Plant Pathology, 132, 24–30.
CFDA. (2017). National food safety standard for maximum levels of mycotoxins in foods. https://www.fas.usda.gov/data/china-china-releases-standard-maximum-levels-mycotoxins-foods
Colás-Medà, P., Nicolau-Lapeña, I., Viñas, I., Neggazi, I., & Alegre, I. (2021). Bacterial spore inactivation in orange juice and orange peel by ultraviolet-c light. Foods, 10(4). https://doi.org/10.3390/foods10040855
Comission Directive 2001/112/EC relating to fruit juices and certain similar products intended for human consumption.
CR(EC)No1881/2006. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs.
Dadachova, E., & Casadevall, A. (2008). Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Current Opinion in Microbiology, 11(6), 525–531. https://doi.org/10.1016/j.mib.2008.09.013
de Capdeville, G., Wilson, C. L., Beer, S. V., & Aist, J. R. (2002). Alternative disease control agents induce resistance to blue mold in harvested “Red Delicious” apple fruit. Phytopathology, 92(8), 900–908. https://doi.org/10.1094/PHYTO.2002.92.8.900
Diao, E., Wang, J., Li, X., Wang, X., Song, H., & Gao, D. (2019). Effects of ozone processing on patulin, phenolic compounds and organic acids in apple juice. Journal of Food Science and Technology, 56(2), 957–965. https://doi.org/10.1007/s13197-018-03561-0
Errampalli, D. (2014). Penicillium expansum (Blue Mold). In Postharvest Decay: Control Strategies. Elsevier. https://doi.org/10.1016/B978-0-12-411552-1.00006-5
FDA. (2005). Food and Drug Administration’s Compliance Policy Guide. ’ Apple juice, apple concentrates and apple juice products - adulteration with patulin’. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-510150-apple-juice-apple-juice-concentrates-and-apple-juice-products-adulteration-patulin
Fenoglio, D., Ferrario, M., Schenk, M., & Guerrero, S. (2020). Effect of pilot-scale UV-C light treatment assisted by mild heat on E. coli, L. plantarum and S. cerevisiae inactivation in clear and turbid fruit juices. Storage study of surviving populations. International Journal of Food Microbiology, 332. https://doi.org/10.1016/j.ijfoodmicro.2020.108767
Food and Drug Administration (FDA). (2002). Guidance for industry: Juice hazard analysis critical control point hazards and controls guidance (p. FDA-2002-D-0298).
Garcia, D., Ramos, A. J., Sanchis, V., & Marín, S. (2011). Intraspecific variability of growth and patulin production of 79 Penicillium expansum isolates at two temperatures. International Journal of Food Microbiology, 151(2), 195–200. https://doi.org/10.1016/j.ijfoodmicro.2011.08.021
Gayán, E., Condón, S., & Álvarez, I. (2014). Biological aspects in food preservation by ultraviolet light: A review. Food and Bioprocess Technology, 7, 1–20. https://doi.org/10.1007/s11947-013-1168-7
Gockmen, V., & Açar, J. (1999). Simultaneous determination of 5-hydroxymethylfurfuraland patulin in apple juice by reversed-phase liquid chromatography. Journal of Chromatographic Annals, 847, 69–74. https://doi.org/10.1016/s0021-9673(99)00133-8
Guerrero, S., Ferrario, M., Schenk, M., Fenoglio, D., & Andreone, A. (2021). Ultraviolet light. In V. M. Gómez-López & R. Bhat (Eds.), Electromagnetic Technologies in Food Science (pp. 127–178). Wiley Blackwell.
Guerrero-Beltran, J. A., & Barbosa-Canovas, G, V. (2004). Review: Advantages and limitations on processing foods by UV light. Food Science and Technology International, 10(3), 137–147. https://doi.org/10.1177/1082013204044359
Gündüz, G. T., & Pazir, F. (2013). Inactivation of Penicillium digitatum and Penicillium italicum under in vitro and in vivo conditions by using UV-C light. Journal of Food Protection, 76(10), 1761–1766. https://doi.org/10.4315/0362-028X.JFP-12-511
Ianiri, G., Pinedo, C., Fratianni, A., Panfili, G., & Castoria, R. (2017). Patulin degradation by the biocontrol yeast Sporobolomyces sp. Is an Inducible Process. Toxins, 9(2), 1–12. https://doi.org/10.3390/toxins9020061
IARC. (2018). International Agency for Research on Cancer. Agents Classified by the IARC Monographs, https://monographs.iarc.fr/agents-classified-by-the-iarc/ (pp. 1–104).
Khademi, O., Zamani, Z., Poor Ahmadi, E., & Kalantari, S. (2013). Effect of UV-C radiation on postharvest physiology of persimmon fruit (Diospyros kaki Thunb.) cv. “Karajr” during storage at cold temperature. International Food Research Journal, 20(1), 247–253.
Khan, S., Dar, A. H., Shams, R., Aga, M. B., Siddiqui, M. W., Mir, S. A., Rizvi, Q. U. eain H., Khan, S. A., & Altaf, A. (2022). Applications of ultraviolet light–emitting diode technology in horticultural produce: A systematic review and meta-analysis. In Food and Bioprocess Technology, 15(3), 487–497. Springer. https://doi.org/10.1007/s11947-021-02742-8
Koutchma, T. (2009). Advances in ultraviolet light technology for non-thermal processing of liquid foods. In Food and Bioprocess Technology, 2(2), 138–155. https://doi.org/10.1007/s11947-008-0178-3
Koutchma, T., Popovic, V., Ros-Polski, V., & Popielarz, A. (2016). Effects of ultraviolet light and high-pressure processing on quality and health-related constituents of fresh juice products. Comprehensive Reviews in Food Science and Food Safety, 15(5), 844–867.
Kowalski, W. (2009). Mathematical modeling of UV disinfection. Ultraviolet Germicidal Irradiation Handbook: UVGI for Air and Surface Disinfection, 51–72. https://doi.org/10.1007/978-3-642-01999-9_3
Kurup, A. H., Patras, A., Pendyala, B., Vergne, M. J., & Bansode, R. R. (2022). Evaluation of ultraviolet-light (UV-A) emitting diodes technology on the reduction of spiked aflatoxin B1 and aflatoxin M1 in whole milk. Food and Bioprocess Technology, 15(1), 165–176. https://doi.org/10.1007/s11947-021-02731-x
La Cava, E. L. M., & Sgroppo, S. C. (2019). Combined effect of UV-C Light and mild heat on microbial quality and antioxidant capacity of grapefruit juice by flow continuous reactor. Food and Bioprocess Technology, 12(4), 645–653. https://doi.org/10.1007/s11947-019-2239-1
Leggott, N. L., & Shephard, G. S. (2001). Patulin in South African commercial apple products. Food Control, 12, 73–76.
Li, B., Chen, Y., Zong, Y., Shang, Y., Zhang, Z., Xu, X., Wang, X., Long, M., & Tian, S. (2019). Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environmental Microbiology, 21(3), 1124–1139. https://doi.org/10.1111/1462-2920.14542
Li, B., Zong, Y., Du, Z., Chen, Y., Zhang, Z., Qin, G., Zhao, W., & Tian, S. (2015). Genomic characterization reveals insights into patulin biosynthesis and pathogenicity in Penicillium species. Molecular Plant-Microbe Interactions, 28(6), 635–647. https://doi.org/10.1094/MPMI-12-14-0398-FI
Lipinski, C. A., Lombardo, F., Dominy, B. W., & Feeney, P. J. (2001). Experimental and computational approaches to estimate solubility and permeability in drug discovery and development q settings. In Advanced Drug Delivery Reviews, 46. www.elsevier.com/locate/drugdeliv
Lovett, J., & Peeler, J. T. (1973). Effect of pH on the thermal destruction kinetics of patulin in aqueous solution. Journal of Food Science, 38, 1094–1095.
Mahato, D. K., Kamle, M., Sharma, B., Pandhi, S., Devi, S., Dhawan, K., Selvakumar, R., Mishra, D., Kumar, A., Arora, S., Singh, N. A., & Kumar, P. (2021). Patulin in food: A mycotoxin concern for human health and its management strategies. Toxicon, 198(January), 12–23. https://doi.org/10.1016/j.toxicon.2021.04.027
Manzocco, L., da Pieve, S., & Maifreni, M. (2011). Impact of UV-C light on safety and quality of fresh-cut melon. Innovative Food Science and Emerging Technologies, 12(1), 13–17. https://doi.org/10.1016/j.ifset.2010.11.006
McKinley, E. R., & Carlton, W. W. (1991). Patulin. In R. P. Sharma & D. K. Salunkhe (Eds.), Mycotoxins and phytoalexins (pp. 191–236). CRC Press.
Morales, H., Marín, S., Rovira, A., Ramos, A. J., & Sanchis, V. (2007). Patulin accumulation in apples by Penicillium expansum during postharvest stages. Letters in Applied Microbiology, 44(1), 30–35. https://doi.org/10.1111/j.1472-765X.2006.02035.x
Morales, H., Sanchis, V., Coromines, J., Ramos, A. J., & Marín, S. (2008). Inoculum size and intraspecific interactions affects Penicillium expansum growth and patulin accumulation in apples. Food Microbiology, 25(2), 378–385. https://doi.org/10.1016/j.fm.2007.09.008
Nassarawa, S. S., Abdelshafy, A. M., Xu, Y., Li, L., & Luo, Z. (2020). Effect of light-emitting diodes (LEDs) on the quality of fruits and vegetables during postharvest period: A review. Food and Bioprocess Technology. https://doi.org/10.1007/s11947-020-02534-6
Ngolong Ngea, G. L., Yang, Q., Castoria, R., Zhang, X., Routledge, M. N., & Zhang, H. (2020). Recent trends in detecting, controlling, and detoxifying of patulin mycotoxin using biotechnology methods. Comprehensive Reviews in Food Science and Food Safety, 19(5), 2447–2472. https://doi.org/10.1111/1541-4337.12599
Nicolau-Lapeña, I., Abadias, M., Viñas, I., Bobo, G., Lafarga, T., Ribas-Agustí, A., & Aguiló-aguayo, I. (2020). Water UV-C treatment alone or in combination with peracetic acid : A technology to maintain safety and quality of strawberries. International Journal of Food Microbiology, 335(Sep), 108887. https://doi.org/10.1016/j.ijfoodmicro.2020.108887
Nicolau-Lapeña, I., Colás-Medà, P., Viñas, I., & Alegre, I. (2022). Inactivation of Escherichia coli, Salmonella enterica and Listeria monocytogenes on apple peel and apple juice by ultraviolet C light treatments with two irradiation devices. International Journal of Food Microbiology, 364, 109535. https://doi.org/10.1016/j.ijfoodmicro.2022.109535
Orlowska, M., Koutchma, T., Grapperhaus, M., Gallagher, J., Schaefer, R., & Defelice, C. (2013). Continuous and pulsed ultraviolet light for nonthermal treatment of liquid foods. Part 1: effects on quality of fructose solution, apple juice, and milk. Food and Bioprocess Technology, 6(6), 1580–1592. https://doi.org/10.1007/s11947-012-0779-8
Orlowska, M., Koutchma, T., Kostrzynska, M., Tang, J., & Defelice, C. (2014). Evaluation of mixing flow conditions to inactivate Escherichia coli in opaque liquids using pilot-scale Taylor-Couette UV unit. Journal of Food Engineering, 120(1), 100–109. https://doi.org/10.1016/j.jfoodeng.2013.07.020
Patras, A., Julakanti, S., Yannam, S., Bansode, R. R., Burns, M., & Vergne, M. J. (2017). Effect of UV irradiation on aflatoxin reduction: A cytotoxicity evaluation study using human hepatoma cell line. Mycotoxin Research, 33(4), 343–350. https://doi.org/10.1007/s12550-017-0291-0
Puel, O., Galtier, P., & Oswald, I. P. (2010). Biosynthesis and toxicological effects of patulin. In Toxins, 2(4), 613–631. https://doi.org/10.3390/toxins2040613
Ran, Y., Qingmin, C., & Maorun, F. (2019). Chlorine dioxide generation method and its action mechanism for removing harmful substances and maintaining quality attributes of agricultural products. Food and Bioprocess Technology, 12(7), 1110–1122. https://doi.org/10.1007/s11947-019-02279-x
Reddy, K. R. N., Spadaro, D., Lore, A., Gullino, M. L., & Garibaldi, A. (2010). Potential of patulin production by Penicillium expansum strains on various fruits. Mycotoxin Research, 26(4), 257–265. https://doi.org/10.1007/s12550-010-0064-5
Riganakos, K. A., Karabagias, I. K., Gertzou, I., & Stahl, M. (2017). Comparison of UV-C and thermal treatments for the preservation of carrot juice. Innovative Food Science and Emerging Technologies, 42, 165–172. https://doi.org/10.1016/j.ifset.2017.06.015
Rios de Souza, V., Popović, V., Warriner, K., & Koutchma, T. (2020). A comparative study on the inactivation of Penicillium expansum spores on apple using light emitting diodes at 277 nm and a low-pressure mercury lamp at 253.7 nm. Food Control, 110(June 2019), 107039. https://doi.org/10.1016/j.foodcont.2019.107039
Rodríguez-Bencomo, J. J., Sanchis, V., Viñas, I., Martín-Belloso, O., & Soliva-Fortuny, R. (2020). Formation of patulin-glutathione conjugates induced by pulsed light: A tentative strategy for patulin degradation in apple juices. Food Chemistry, 315. https://doi.org/10.1016/j.foodchem.2020.126283
Sajid, M., Mehmood, S., Yuan, Y., & Yue, T. (2019a). Mycotoxin patulin in food matrices: occurrence and its biological degradation strategies. Drug Metabolism Reviews, 51(1), 105–120. https://doi.org/10.1080/03602532.2019a.1589493
Saleh, I., & Goktepe, I. (2019). The characteristics, occurrence, and toxicological effects of patulin. Food and Chemical Toxicology, 129, 301–311. https://doi.org/10.1016/j.fct.2019.04.036
Salomao, B. C. M., Aragao, G. M. F., Churey, J. J., & Worobo, R. W. (2008). Efficacy of sanitizing treatments against Penicillium expansum inoculated on six varieties of apples. Journal of Food Protection, 71(3), 643–647. http://meridian.allenpress.com/jfp/article-pdf/71/3/643/1681868/0362-028x-71_3_643.pdf
Seltsam, A., & Müller, T. H. (2011). UVC irradiation for pathogen reduction of platelet concentrates and plasma. Transfusion Medicine and Hemotherapy, 38(1), 43–54. https://doi.org/10.1159/000323845
Shama, G., & Alderson, P. (2005). UV hormesis in fruits: A concept ripe for commercialisation. Trends in Food Science and Technology, 16(4), 128–136. https://doi.org/10.1016/j.tifs.2004.10.001
Shao, S., Zhou, T., & McGarvey, B. D. (2012). Comparative metabolomic analysis of Saccharomyces cerevisiae during the degradation of patulin using gas chromatography-mass spectrometry. Applied Microbiology and Biotechnology, 94(3), 789–797. https://doi.org/10.1007/s00253-011-3739-8
Shephard, G. S., & Leggott, N. L. (2000). Chromatographic determination of the mycotoxin patulin in fruit and fruit juices. Journal of Chromatography A, 882(1–2), 17–22. https://doi.org/10.1016/S0021-9673(99)01341-2
Syamaladevi, R. M., Adhikari, A., Lupien, S. L., Dugan, F., Bhunia, K., Dhingra, A., & Sablani, S. S. (2015). Ultraviolet-C light inactivation of Penicillium expansum on fruit surfaces. Food Control, 50, 297–303.
Syamaladevi, R. M., Lu, X., Sablani, S. S., Insan, S. K., Adhikari, A., Killinger, K., Rasco, B., Dhingra, A., Bandyopadhyay, A., & Annapure, U. (2013). Inactivation of Escherichia coli population on fruit surfaces using ultraviolet-C light: Influence of fruit surface characteristics. Food and Bioprocess Technology, 6(11), 2959–2973. https://doi.org/10.1007/s11947-012-0989-0
Syamaladevi, R. M., Lupien, S. L., Bhunia, K., Sablani, S. S., Dugan, F., Rasco, B., Killinger, K., Dhingra, A., & Ross, C. (2014). UV-C light inactivation kinetics of Penicillium expansum on pear surfaces: Influence on physicochemical and sensory quality during storage. Postharvest Biology and Technology, 87, 27–32. https://doi.org/10.1016/j.postharvbio.2013.08.005
Tannous, J., Keller, N. P., Atoui, A., El Khoury, A., Lteif, R., Oswald, I. P., & Puel, O. (2018). Secondary metabolism in Penicillium expansum: Emphasis on recent advances in patulin research. Critical Reviews in Food Science and Nutrition, 58(12), 2082–2098. https://doi.org/10.1080/10408398.2017.1305945
Tikekar, R. V., Anantheswaran, R. C., & Laborde, L. F. (2014). Patulin degradation in a model apple juice system and in apple juice during ultraviolet processing. Journal of Food Processing and Preservation, 38(3), 924–934. https://doi.org/10.1111/jfpp.12047
Torres, R., Valentines, M. C., Usall, J., Viñas, I., & Larrigaudiere, C. (2003). Possible involvement of hydrogen peroxide in the development of resistance mechanisms in “Golden Delicious” apple fruit. Postharvest Biology and Technology, 27(3), 235–242. https://doi.org/10.1016/S0925-5214(02)00110-2
Tournas, V. H. (2005). Spoilage of vegetable crops by bacteria and fungi and related health hazards. Critical Reviews in Microbiology, 31(1), 33–44. https://doi.org/10.1080/10408410590886024
Valero, A., Begum, M., Leong, S. L., Hocking, A. D., Ramos, A. J., Sanchis, V., & Marín, S. (2007). Effect of germicidal UVC light on fungi isolated from grapes and raisins. Letters in Applied Microbiology, 45(3), 238–243. https://doi.org/10.1111/j.1472-765X.2007.02175.x
Veber, D. F., Johnson, S. R., Cheng, H. Y., Smith, B. R., Ward, K. W., & Kopple, K. D. (2002). Molecular properties that influence the oral bioavailability of drug candidates. Journal of Medicinal Chemistry, 45(12), 2615–2623. https://doi.org/10.1021/jm020017n
Vidal, A., Ouhibi, S., Ghali, R., Hedhili, A., de Saeger, S., & de Boevre, M. (2019). The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food and Chemical Toxicology, 129, 249–256. https://doi.org/10.1016/j.fct.2019.04.048
Vignali, G., Gozzi, M., Pelacci, M., & Stefanini, R. (2022). Non-conventional stabilization for fruit and vegetable juices: Overview, technological constraints, and energy cost comparison. Food and Bioprocess Technology, 1729–1747. https://doi.org/10.1007/s11947-022-02772-w
Viñas, I., Usall, J., Teixidó, N., & Sanchis, V. (1995). Presence of funghi strains resistant to fungicides in the post-harvest of fruit in Lleida. II Congreso Ibérico De La Sociedad Española De Ciencias Hortícolas, 91(1), 6–20.
Ware, G. M., Thorpe, C. W., & Pohland, A. E. (1974). Liquid chromatographic method for determination of patulin in apple juice. Journal of AOAC, 57, 111–113.
Woo, H. J., Kang, J. H., Lee, C. H., & Song, K. B. (2020). Inactivation of Listeria monocytogenes, Escherichia coli O157:H7, and pre-existing bacteria on spinach by combined treatment of Cudrania tricuspidata leaf extract washing and ultraviolet-C irradiation. Food and Bioprocess Technology, 13(7), 1229–1239. https://doi.org/10.1007/s11947-020-02476-z
Yamaga, I., & Nakamura, S. (2019). Changes in fungal development, scoparone accumulation, and natural disease infection after ultraviolet-C irradiation in Satsuma mandarin “Aoshima Unshu” fruit. Tropical Agriculture and Development, 63(4), 204–209.
Zetina-Serrano, C., Rocher, O., Naylies, C., Lippi, Y., Oswald, I. P., Lorber, S., & Puel, O. (2020). The brla gene deletion reveals that patulin biosynthesis is not related to conidiation in Penicillium expansum. International Journal of Molecular Sciences, 21(18), 1–32. https://doi.org/10.3390/ijms21186660
Zhu, Y., Koutchma, T., Warriner, K., & Zhou, T. (2014). Reduction of patulin in apple juice products by UV light of different wavelengths in the UVC range. Journal of Food Protection, 77(6), 963–971. https://doi.org/10.4315/0362-028X.JFP-13-429
Acknowledgements
The authors are thankful to R. Magalhaes-Gomez for her technician support. The authors acknowledge the UV-Consulting Peschl España for providing the UV-C laboratory scale equipment used in the experiments and Dallan, S.A. Moleva for the contribution with apple juice. J.J. R-B acknowledges the support of the Community of Madrid/FEDER program [S2018/BAA-4393, AVANSECAL II-CM] for the nLC-MS analysis.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was funded by the Spanish Ministry of Economy, Industry and Competitiveness (MINECO), with a postdoctoral orientation period grant [BES 2017 079779] (I. Nicolau-Lapeña).
Author information
Authors and Affiliations
Contributions
Iolanda Nicolau-Lapeña and Juan José Rodríguez Bencomo designed and performed the experiments, analyzed the data, and wrote the main manuscript text. Pilar Colás-Medà and Isabel Alegre designed the experiments. Isabel Alegre was in charge of overall direction and planning. Inmaculada Viñas and Vicente Sanchís conceived of the presented idea and supervised the project. All authors discussed the results and commented on the manuscript, contributing to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Ultraviolet light C reduced more than 99.9 % P. expansum conidia on apple surface.
• Patulin production by P. expansum in apples decreased by 8.8 and 35.1 kJ m−2 UV-C254nm.
• Patulin in apple juice was reduced 98.6 % after 450.6 kJ m−2 UV-C254nm treatment.
• Degradation products of patulin after UV-C254nm treatment were tentatively identified.
• Degradation products did not show greater toxicity or biological activity than patulin.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nicolau-Lapeña, I., Rodríguez-Bencomo, J.J., Colás-Medà, P. et al. Ultraviolet Applications to Control Patulin Produced by Penicillium expansum CMP-1 in Apple Products and Study of Further Patulin Degradation Products Formation and Toxicity. Food Bioprocess Technol 16, 804–823 (2023). https://doi.org/10.1007/s11947-022-02943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02943-9