Abstract
Cell structure modification techniques have the potential to improve curcuminoid recovery in Curcuma longa. In this study, different pre-treatments such as high hydrostatic pressure (HPP, high pressure processing), ultrasound (US), pulsed electric field (PEF), and ohmic heating (OH) were used on dried C. longa before aqueous extraction at pH 2.0, 5.0, and 8.0. The released curcuminoids, cell disintegration index (Zp), particle size distribution (PSD), and color (CIE L*, a*, b*) were used to evaluate the different pre-treatment impacts on plant structure and extract properties. In untreated turmeric, the highest amount of released curcuminoids (3.89 mg/g dry matter) was obtained after extraction for 30 min at 95° in the aqueous phase. After pre-treatments, the acidic conditions showed a considerable improvement in curcuminoid recovery; PEF, HPP, and OH improved the curcuminoid recovery by 3.39-, 3.13-, and 1.24-fold, respectively; while US did not lead to an increased release of curcuminoids compared to the untreated material. The highest curcuminoid recovery (with PEF and extraction at pH 5.0) was 6.6% w/w of the total curcuminoids. The non-thermal pre-treatments have less impact on the extract’s color compared to the extraction pH, with alkaline conditions reducing the lightness of the extract.
Similar content being viewed by others
Introduction
Curcumin was first isolated by Vogel and Pelletier (1815) and was known as an orange-yellow crystalline powder. Curcumin is the most bioactive compound in Curcuma longa (Kiamahalleh et al., 2016). Numerous studies showed that curcumin is a potent antioxidant compound with antimicrobial and anti-inflammatory properties (Anand et al., 2008; Ramalingam & Ko, 2014; Zheng et al., 2019). It was examined that curcumin also has beneficial effects in treating cancer, diabetes, obesity, Alzheimer’s disease, and stroke (Priyadarsini, 2014; Zheng et al., 2019). In food science, curcumin is mainly used as a coloring, flavoring, and preservative agent in functional foods or beverages (Aggarwal et al., 2006; Joshi et al., 2013). Numerous publications about curcumin extraction reveal challenges resulting from its poor dispersibility in aqueous solutions, low chemical stability, and ease of degradation in undesirable conditions (intense light, temperature, and oxygen) (Gordon et al., 2015; Lestari & Indrayanto, 2014; Niu et al., 2012; Schieffer, 2002).
Curcumin is a non-polar compound, so it dissolves quickly in an organic solvent such as hexane, acetone, and ethanol. Many studies use these organic solvents for selective extraction of curcumin (Priyadarsini, 2014). However, due to increasing consumer demand for organic-solvent-free products, strategies for the aqueous extraction of polyphenols have gained new attention (Ameer et al., 2017; Chemat et al., 2015). Furthermore, the application of modern techniques to curcumin extraction by aqueous phase is a new and promising work to provide an understanding of the potential approaches in the food industry.
An aqueous extraction broadens the application of extracts in the pharmacy and food industry. However, curcumin has low solubility and low stability in the aqueous phase (Gordon et al., 2015; Niu et al., 2012). In the research of Rahman et al. (2009), curcumin solubility in the aqueous phase is 231 μg/ml at pH 4.0 and increases to 482 μg/ml at pH 8.0. Furthermore, the complex plant matrix of the material is a huge barrier that prevents the mass transfers and diffusion of curcumin during the extraction. A higher extraction yield is often associated with the destruction of cell walls and other structural barriers, facilitating the mass transfer of the solvent into the plant material.
Unconventional techniques are ultrasound (US) (Lee et al., 2014; Pérez-Elvira et al., 2009), high hydrostatic pressure (HPP, high pressure processing) (George et al., 2018; Yusoff et al., 2017), pulsed electric field (PEF) (Gulzar & Benjakul, 2020; Parniakov et al., 2015; Yu et al., 2016), and ohmic heating (OH) (Gavahian et al., 2020; Pereira et al., 2016, 2020). These techniques showed a high impact on cell structure modification and enhanced the active compound’s recovery. So far, these pre-treatments have not been investigated in one study with a comparable experimental setup. Despite the different mechanisms of impact, the benefit the different technologies have in common is the rapid treatment, which could reduce curcumin exposure time to undesired conditions.
Besides cell wall disintegration, the curcumin’s physical state is the crucial factor affecting mass transfer and diffusion; the mechanism of changing the physical state is based on the molecule’s solubility changes under different pH conditions (Peng et al., 2019). Numerous studies concluded that curcumin is insoluble in the acidic aqueous phase and soluble in the polar and non-polar aqueous phase at alkaline conditions (Lestari & Indrayanto, 2014). In polar liquids or solids, curcumin is often found in crystalline form, which can change depending on the pH of the solvent. Compared to its dissolved form, curcumin is more stable towards degradation in the crystalline state (Kiamahalleh et al., 2016; Schieffer, 2002).
The current research focused on comparing the efficacy of different cell disintegration techniques on curcuminoid recovery during extraction at different pH conditions. Cell disintegration index, particle size distribution, released curcumin, and extract’s colors were evaluated. This research’s main objective is to establish an aqueous extraction process to enhance curcuminoid recovery without using an organic solvent. The safe product obtained is used as a supplement in the food industry.
Material and Method
Materials
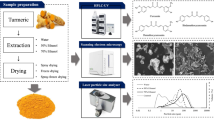
The fresh rhizomes of turmeric (C. longa) were obtained from a local market in Vienna, Austria. The fresh material was cut into 1 cm pieces and dried at 50 °C for 72 h by drying oven. Afterward, dried turmeric was frozen by liquid nitrogen in 5 min, milled to the powder, and sieved with pore size 2 mm for getting homogenous grain sizes. The mean particle size of dried powder was 357.23 ± 12.24 µm. All solvents and chemicals used for the extraction were of analytical grades (Merck Specialities Private Limited, Germany).
Dry Matter Content
Dry matter content was determined for all raw materials. The dry matter content was used to calculate the total curcumin recovery based on a dry basis. Around 1 g of turmeric powder was weighed in an aluminum pan and dried at 103 °C for 4 h. The percentage between sample weight after and before the drying is the dry matter content of the sample.
Pre-treatment
The dried turmeric powder was mixed with deionized water with a ratio of 1:10 before the pre-treatment. The procedure of pre-treatment is shown below:
High-Pressure Pre-treatment
The HPP was performed in a high-pressure unit (FF725, Nova Swiss Sarl, Cesson, France). The sample was filled in PET bottles and placed into plastic bags. After that, the bags were vacuum-sealed. Finally, the bags were placed in a high‐pressure vessel filled with water and subjected to 100, 200, or 300 MPa for 5 min. These conditions were chosen based on the recommended pressure on fruit (Yucel et al., 2010) and the trial experiments (data not shown). The temperature was measured continuously during the treatment, the initial temperature was 20 °C, and the treatment temperature ranged from 16 to 25 °C. Pressure build-up to the desired pressure took 30–50 s. Based on the first law of thermodynamics and incorporation of the isothermal compressibility, the volumetric work (kJ/kg) from pressure A (0 MPa) to pressure B can be calculated on Eq. (1):
where p is the working pressure (Pa), v is the specific volume (cm3/g), and \({\beta }_{T}\) is the isothermal compressibility (Pa−1). All of the parameters were collected at 20 °C.
The energy input was the basis for comparing different treatment intensities. The different pressure levels of 100, 200, and 300 MPa correspond to the following volumetric work of compression: 4.7 kJ/kg, 9.3 kJ/kg, and 13.9 kJ/kg, respectively (see Table 1).
Ultrasound Pre-treatment (US)
The US pre-treatment was performed using an ultrasound generator and a sonotrode (UP200St, Hielscher ultrasonic processor, Germany). The dried turmeric was mixed with deionized H2O in the flask at the ratio of 1:10; then ultrasound was applied to the system using the sonotrode immersed in the sample approximately 10 cm. The ultrasonic power was set at 50 W, 100 W, 150 W, and the residence time was 2 min for each treatment. The setting conditions were chosen based our previous research (Le-Tan et al., 2021). The flask was put in an ice container to avoid temperature increase during the treatment. Because of that, the maximum temperature of the material was maintained lower than 10 °C during the treatment.
The specific energy input was the basis for comparing different treatment intensities. The treatment at 50 W, 100 W, and 150 W were converted into 182 kJ/kg, 346 kJ/kg, and 546 kJ/kg, respectively (see Table 1).
Pulsed Electric Field Pre-treatment
The PEF effect was investigated by using a batch PEF system (DIL, Germany). The PEF treatment chamber had an electrode distance of 1 cm, the ratio of dried turmeric: deionized H2O was 1: 10. Voltage and current applied to the treatment chamber were monitored using a high voltage probe (Tektronix P6015A, Beaverton USA) and a current transformer (model no. 0.5–0.1 W, Stangenes Industries Inc., Palo Alto, CA, USA) connected to a 2-channel digital oscilloscope (Tektronix TBS 1102B-EDU, Beaverton, USA). The field strength was set to 2 kV/cm, the voltage was 2 kV, and the pulse width was 49 µs. The number of applied exponential decay pulses was 10, 20, and 50. The number of pulses was chosen based on the desired total specific energy input obtained from the preliminary experiment (data not shown) and according our previous study on curcuminoid extraction (Le-Tan et al., 2021):
The specific energy input was the basis for comparing different treatment intensities. The treatments at 10 pulses, 20 pulses, and 50 pulses were converted into 0.3 kJ/kg, 0.6 kJ/kg, and 1.5 kJ/kg, respectively (see Table 1).
Ohmic Heating Pre-treatment (OH)
The OH was performed using the same treatment chamber but a different generator (DIL, Germany) to apply the electric field (1000 V, 0.3 kW), resulting in electric field strength of 1000 V/cm. The target temperature was set at 50 °C, 70 °C, and 95 °C, and the needed time to increase the temperature from the beginning (~ 20 °C) to the desired temperature was 25 ± 2 s, 60 ± 7 s, and 119 ± 10 s, respectively. The temperature of 50 °C is a gentle temperature, which could not affect bioactive compounds. According to Maskooki and Eshtiaghi (2012), 70 °C is the temperature that starts the thermal effect on the plant cell, while turmeric starch could be gelatinization at 95 °C.
The specific energy input was the basis for comparing different treatment intensities. The treatment at 50 °C, 70 °C, and 95 °C were converted into 105 kJ/kg, 188 kJ/kg, and 272 kJ/kg, respectively (see Table 1).
Curcuminoid Aqueous Extraction
After pre-treatment, water was removed by centrifugation at 1700 relative centrifugal force (RCF) for 5 min. One gram of sample was weighted in a 10 mL flask tube. Three buffer solutions for aqueous extraction were prepared: phosphate buffer solution pH 2.0, acetate buffer solution pH 5.0, phosphate buffer solution pH 8.0 according to the European Pharmacopoeia 7.0 (Commission et al., 2010) method. Then, 8 mL of buffer solution was added by a volumetric pipette at room temperature (~20 °C). The aqueous extraction was conducted in a shaking water bath with a light inhibiting cover.
The water bath was set with shaking speed at 100 rounds per minute (rpm). The extraction was conducted from 15 to 300 min, at two different temperatures of 50 °C and 95 °C. After shaking, the tubes were cooled down immediately in an ice bath. Then, the flask tube was centrifuged (1700 relative centrifugal force (rcf), 5 min) to get the extracts for further analysis. The untreated sample (without pre-treatment) was prepared and extracted in the same conditions.
Determination of Cell Disintegration Index
In order to evaluate the efficiency of pre-treatment on curcumin recovery, cell disintegration index (CDI) mainly was used to claim the damage of turmeric cells before and after the pre-treatments (Le-Tan et al., 2021; Luengo et al., 2016; Maskooki & Eshtiaghi, 2012). The mechanism of CDI measurement is the difference of impedance measured at the different current frequencies (Ando et al., 2014). The difference in material impedance before and after the treatment shows the damage of the plant cell (Donsì et al., 2010).
The cell disintegration index (Zp) was determined by measuring the pre-treated sample’s electrical conductivity with an impedance analyzer (Sigma Check, EloCheck, Berlin, Germany); the electrical current goes through the sample when connected to a 12 V DC source. The sample was put in a cylindrical tube (d = 10 mm, l = 10 mm). The tube was placed between two stainless steel electrodes. There were 14 different frequency levels applied from 1.38 kHz to 11.2 MHz. The Zp was calculated according to Fauster et al. (2018) using the impedance value at 5.5 and 1400 kHz. The untreated sample was considered the control sample.
According to Angersbach et al. (1999), the plant disintegration index (\({p}_{0}\)) is described by Eq. (3):
where \({p}_{0}\) is the measured electrical conductivity value and the subscripts i and s refer to the conductivities of untreated (initial) and treated (damaged) tissue, respectively; the subscripts l and h refer to low frequency and high frequency range. In theory, \({p}_{0}\) = 0 for an intact tissue and \({p}_{0}\) = 1 for a maximally disintegrated tissue. In the current research, the value of \({p}_{\mathrm{max}}\) was estimated by measurements of freeze-thawed tissue’s electrical conductivity for two rounds (Le-Tan et al., 2021). After such treatment, the electrical conductivity of tissue attained its maximal value.
Quantification of Curcumin
The curcuminoids in the raw materials used in this study consist of 14.37%w/w dry basis (6.17%w/w dry basis of BDMC, 2.44%w/w dry basis of DMC, and 5.76%w/w dry basis of curcumin) as analyzed and reported in our previous study (Le-Tan et al., 2021). In order to track the curcuminoid recovery in this study, the spectrophotometry method was used to determine the curcuminoid content (TC). Sediment separation in watery extracts was accelerated by centrifuging the dispersion for 5 min (1700 rcf). The supernatant was diluted ten times with ethanol (96%). The UV–Vis spectra (350–750 nm) of the diluted extracts were recorded in a UV–Vis spectrophotometer (UV-1800, Shimadzu, Japan) and the Absorbance at 424 nm corrected by the absorbance at 525 nm was used for the calculation of the curcuminoid content based on a previously prepared calibration curve (n = 6; R2 = 1.0000) of pure curcumin absorbance in ethanol was used to calculate the curcuminoid content in mg/g. The curcuminoid content is expressed as curcumin equivalent in the whole study.
Particle Size, Surface Area, and Span Value
The particle size distribution (PSD) was determined using a laser diffraction system (LA-960, Horiba, Japan) equipped with a liquid and a dry dispersion unit. PSD was measured according the Haas et al. (2019) method with modifications. The particle size of the sample was measured in the liquid dispersion system filled with water (150 mL); ultrasound for 15 s was used to avoid an agglomeration of the particles. Agitation was running during the measurement. The span value calculated as (d90 − d10)/d50 indicates how far the 10 percent and 90 percent points are apart from the midpoint.
Color (CIE L*, a*, b*) Measurement
The color measurement was performed as described by Skrede (1985) with some modifications. The aqueous extracts were centrifuged (5 min, 1700 rcf), and the supernatant was measured in a 1 cm path-length quartz cuvette using 1800-UV spectrophotometer (Shimadzu, Sesakusho Ltd., Kyoto, Japan). CIE L*, a*, b* values were calculated from the transmission spectra between 360 and 760 nm (1 nm intervals). The color L*, a*, b* was calculated by UVPC Color Analysis, Personal Spectroscopy Software, Version 3.12.
Morphology
Curcuminoid crystal in the extract was visualized using a light microscope (Olympus BX51, Tokyo, Japan) equipped with a camera (Olympus XC50, Tokyo, Japan). The image was measured and processed using the cellSens Dimension software (version 1.12), enabling size measurement.
Statistical Analysis
All pre-treatments and analyses were conducted in triplicates. Statistical analysis was performed using Statgraphics Centurion XVII, version 17.1.04 (Statpoint Technologies, Inc., Warrenton, VA, USA). One-way ANOVA (analysis of variance with α = 0.05) and Fisher’s least significance test was used to establish the significance of differences among the mean values. Results are expressed as mean ± standard deviations of three single determinations.
Result and Discussion
Regarding polyphenol extraction, a few studies report that the temperature range of 70–80 °C and treatment time longer than 10 min starts to generate an impact on plant cells (Ade-Omowaye et al., 2001; Schreider, 1968). Bioactive components could be more stable with the extraction temperature at 50 °C; however, gentle temperature could limit the extraction yield (Ade-Omowaye et al., 2001). The results in the current study are in agreement with the previous conclusion from the literature. At 50 °C, curcumin recovery remained lower than 2 mg/g in the pH range from 2.0 to 8.0 (Fig. 1a).
In order to have good comparative results with the extraction at high temperatures, the experiments were conducted at 95 °C in the same pH range (2.0 to 8.0). According to Shirsath et al. (2017), increasing extraction temperature could improve the aqueous medium’s physical properties, including surface tension, solubility, viscosity, and diffusivity.
The result indicates that in the acidic condition (pH 2.0), the released curcumin to the aqueous phase at 95 °C was 3.3-fold higher than that at 50 °C. While at pH 5.0, this value is 1.49-fold higher (Fig. 1a, b). With this regard, a possible hypothesis for the mechanism could be as follows: at 50 °C, the mass transfer mainly takes place by gentle diffusion, which has a low effect on crystalline curcumin at a low pH value (Kharat et al., 2017). While at high temperatures (95 °C), the mass transfer improved significantly because of the applied temperature near the aqueous phase’s boiling point. That could be the reason for the dramatic increase in released curcumin at pH 2.0 (Fig. 1).
However, the weak alkaline condition (pH 8.0) had a different impact on curcumin recovery. At 50 °C, the extraction under pH 8.0 condition performs better than the extractions at pH 2.0 and 5.0 (Fig. 1a). According to Kaminaga et al. (2003), curcumin increases solubility when the pH conditions change from acidic to alkaline, leading to the higher diffusion of curcumin under weak alkaline pH conditions. Interestingly, although higher soluble at alkaline pH, curcumin also reduces bioavailability and stability under this condition (Niu et al., 2012; Osorio-Tobón et al., 2014).
Based on the observations, it has been established that, in low extraction temperature (50 °C), weak alkaline pH could be used for fast diffusion and mass transfer of curcumin (shorter than 15 min). However, more extended time could reduce the curcumin recovery due to prolonged exposure to light, and oxygen can trigger curcumin degradation. Moreover, after 15 min of shaking at different pH, the released curcumin was not improved at 50 °C in comparison with 95 °C (Fig. 1a, b). The shaking time was continually extended to investigate further the effect of the shaking time on curcumin recovery. When the shaking time increased to 30 min, the released curcumin at 95 °C increased rapidly at pH 2.0 (Fig. 1b). The results revealed that the mass transfer improved immensely because it almost reached the aqueous phase’s boiling point. The result indicates that at pH 2.0, the released curcumin was much higher than pH 5.0 and 8.0.
In terms of polyphenol extraction, high-temperature treatment was not recommended (Ameer et al., 2017). However, in the dark condition, curcumin showed to be stable at temperatures lower than 100 °C (Lestari & Indrayanto, 2014). As opposed to extraction at 50 °C, at 95 °C, the released curcumin in the aqueous phase increased continuously from 15 – 30 min. Compared with the extraction at 50 °C, the released curcumin of 30 min at 95 °C was 330% and 149% higher at pH 2.0 and 5.0, respectively. The result showed that acidic pH (2.0 and 5.0) improved the curcumin recovery after 30 min of shaking at 95 °C, while alkaline condition (pH 8.0) did not show the improvement.
Noteworthy, curcumin degrades faster in alkaline and neutral pH conditions than acidic conditions. With this regard, a mechanism was reported by Jovanovic et al. (1999). At alkaline condition, the destruction of the conjugated diene structure occurs due to the loss of the proton from the activated carbon atom in the central heptadienone linkage in the curcumin molecule, which makes the curcumin molecule sensitive to water. Degradation products of curcumin are p-hydroxybenzoic acid, ferulic aldehyde, p-hydroxybenzaldehyde, vanillic acid, vanillin, and ferulic acid (Schieffer, 2002).
In scientific literature, many studies were incomplete to report the effect of high temperature on curcumin recovery with long shaking times at different pH conditions. In order to gain further insight into the effect of different pH conditions on released curcumin, the shaking time was extended to 5 h. It can be seen from Fig. 1b that the extent of shaking time to 5 h, curcumin recovery reduced. In detail, the curcumin content in the extracts was followed the order pH 2.0 > pH 5.0 > pH 8.0 (Fig. 1b); it was found that when prolonging the extraction time, the acidic condition shows the better extraction ability or the better curcumin retain ability. Compared with the extraction peak at 30 min, after 5 h, the amount of curcumin retention reduced by 9% at pH 2.0 instead of 28% and 33% reduction at pH 5.0 and 8.0, respectively. Based on the above observations, it can be stated that pH 5.0 improved curcumin recovery in a short time (less than 30 min); however, pH 2.0 keeps the curcumin stable for a longer time.
Effects of Cell Disintegration Pre-treatment on Released Curcumin at Different pH
In the conducted experiments, the variability of pH conditions on the curcumin recovery from non-treated samples was compared with pre-treated samples. At alkaline pH, curcumin exists in enol form and changes to keto form in acidic pH; these changes increase the curcumin polarity in alkaline conditions and reduce the polarity in acidic conditions (Lestari & Indrayanto, 2014). As a result, in the aqueous phase at alkaline conditions, the mass transfer of curcumin is faster, but the extraction time also should be limited because curcumin degrades into ferulic acid and feruloylmethane in alkaline pH conditions (Brittain, 2020). The effect of aqueous phase pH on released curcumin will be further discussed below. The experiments were established for turmeric material with and without pre-treatment.
High-Pressure Pre-treatment
Levels of curcumin released after the high-pressure pre-treatment (HPP) are shown in Fig. 2a. With the pre-treatment, the released curcumin improved by 1.6-fold (at pH 2.0), 3.3-fold (at pH 5.0), and 4.7-fold (at pH 8.0) compared with the untreated samples. During HPP treatment, the pressure disrupted the plant cell wall and membrane and enhanced the mass transfer (Huang et al., 2013).
Effect of pre-treatment and pH condition on released curcumin by aqueous extraction. (In each pre-treatment, means with a different letter are significantly different (p < 0.05). High-pressure pre-treatment (a), ultrasound pre-treatment (b), PEF pre-treatment (c), OH pre-treatment (d). After pre-treatment, extraction was conducted at 95 °C in 30 min)
At 300 MPa, the CDI value was 60% higher than that at 100 MPa, and the particle size was reduced by 28% (Table 1). It can be seen from Table 1 that when increasing the pressure, the particle size tends to decrease, and the cell disruption index increases. These parameters could enhance the mass transfer of curcumin to the aqueous phase. According to ), high pressure could collapse and wrap the cell wall, affect the cell textural properties, and result in irreversible structural changes. The applied pressure should be high enough to get a sufficient impact on the cell wall; typically, it must exceed the critical value, which is specific for each plant matrix depending on the cell structure and stability (Khan et al., 2019). In the onion processing research, this critical pressure was 100 MPa (Butz et al., 1994). In the current study, 200 MPa treatment was not high enough to increase the cell destruction compared with 100 MPa treatment (Table 1).
The results show that pre-treatment at 300 MPa, the mean particle size did not change compared to 200 MPa (Table 1), while the surface area and CDI of the material increased by 37% and 67%, respectively (Table 1). After the holding and rapid release of high pressure, some massive particles were broken into smaller particles. This phenomenon may not change the bulk’s mean size, but it changed the size distribution width (express as span value) and increased the total surface area (Table 1). At the same pH condition, the curcumin recovery did not improve when increasing the pressure from 100 to 300 MPa. The results revealed that the released curcumin was affected mainly by the pH condition rather than the increase of pressure.
In general, the extraction at pH 5.0 performed well compared to pH 2.0 and 8.0 (Fig. 2a). The result showed that pH 5.0 is a balancing condition between the solubility and stability of curcumin.
Ultrasound Pre-treatment
The impact of ultrasound on total curcumin recovery is shown in Fig. 2b. In general, the increasing US energy did not increase the curcumin released at pH 2.0 and pH 5.0, while pH 8.0 improved curcumin recovery. At pH 8.0, the curcumin recovery increased by 57% compared to the untreated sample.
The results revealed that ultrasound treatment considerably reduces the particle size when increasing the energy input. The mean particle size under US-364 and US-546 treatments was reduced by 6% and 55%, respectively, compared to the untreated sample (Table 1). Nevertheless, the cell disintegration index did not significantly change when increasing the ultrasound power (Table 1). In the previous study, Shirsath et al. (2017) reported that ultrasound could easily break the fragile links between particles and reduce particle size. The primary mechanism of ultrasound is forming cavitation bubbles, which could impact the plant cell (Ordóñez-Santos et al., 2015), but ultrasound’s compression and tension effect did not last long after stopping the treatment. Based on these observations, the possible hypothesis is that ultrasound energy input at 182 kJ/kg and 364 kJ/kg in 5 min can reduce powder particle size but not efficiently modify the cell wall structure. In order to optimize the extraction yield of sensitive compounds, ultrasound should be used at adequate intensity, high enough to improve the recovery but not trigger the degradation of bioactive molecules (Hadi et al., 2015; Shirsath et al., 2017). In addition, it could be considered to apply ultrasound during the extraction rather than as a pre-treatment in order to benefit from the mass transfer improvement (Vilkhu et al., 2008).
In contrast to other pre-treatments in this study, ultrasound reduced the released curcumin when increasing the ultrasound input energy (at pH 5.0). Furthermore, pH 5.0 did not show the better curcumin recovery than pH 2.0 and pH 8.0 (Fig. 2b); this was unexpected because at pH 5.0; HPP, PEF, and OH all showed a higher recovery than at pH 2.0 and 8.0 (Fig. 2a, c, d). The possible hypothesis for this might be explained by the report of Pingret et al. (2013); Pringet et al. stated that acoustic cavitation could produce OH− and H+ radicals, which mainly impact sensitive compounds and the formation of degradation molecules. These degradation molecules can subsequently trigger a degradation chain reaction and reduce the presence and thus the extraction yield of the sensitive compound. Furthermore, the exceptionally high impact of cavitation bubble collapsing during ultrasound (5000 °K and 5000 atm) could significantly accelerate the chemical reactivity (Flint & Suslick, 1991; Suslick et al., 1999, 2011). Based on the observations, it has been established that ultrasound reduced the particle size and increased the surface area of the material; however, the high intensive impact on the materials did not improve the curcumin recovery.
Pulsed Electric Field Pre-treatment
In order to improve the curcumin recovery, PEF could be used as a pre-treatment before extraction (Vorobiev & Lebovka, 2006). In the research of Le-Tan et al. (2021), the authors used PEF as a pre-treatment before rapid shaking extraction by ethanol; the curcumin recovery increased by 48.3%. In the current study, PEF is used as pre-treatment before aqueous extraction. The effect of PEF on the released curcumin was shown in Fig. 2c. It is notable that the materials with PEF pre-treatment demonstrated higher released curcumin in the aqueous phase. The curcumin recovery from PEF pre-treatment was improved 2.05-fold, 3.39-fold, and 3.84-fold at pH 2.0, 5.0, and 8.0, respectively (Fig. 2c). Furthermore, at pH 2.0 condition, the PEF pre-treatment showed a significant improvement of released curcumin compared to the untreated sample (105%), while released curcumin in HPP and OH was 66% and 29% higher than the untreated sample, respectively.
In Table 1, it can be observed that when the PEF energy input reaches 1.5 kJ/kg, the CDI increases dramatically by 90% compared to the samples treated at 0.3 kJ/kg.
In terms of PEF pre-treatment, the particle size distribution and the surface area did not change, although the CDI value increased (Table 1). With this regard, the explanation could be based on the reports from previous studies on PEF; the primary mechanism of PEF is electropermeabilization, which can increase the impedance of material but does not damage the cell wall (Teissie et al., 2005) by creating temporary or permanent pores (Barba et al., 2016) while maintaining the initial particle size (Han et al., 2009) of the material.
The obtained results established that PEF increased the released curcumin under acidic pH conditions (pH 2.0 and 5.0) but showed less efficiency weak alkaline pH conditions (pH 8.0) (Fig. 2).
Ohmic Heating Pre-treatment
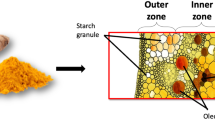
In the OH pre-treatment, the material took 25, 60, and 119 s to heat from 20 to 50 °C, 70 °C, and 95 °C in the core temperature, respectively. Mean particle size increased by 8.8% when increasing the OH temperature from 50 to 95 °C. It was found that the CDI raised by 20% when increase the OH temperature from 50 to 95 °C (Table 1). The high amount of turmeric starch could explain the increase of particle size after the pre-treatment due to gelatinization effects. The primary ingredient fraction in turmeric is the carbohydrate, which reaches over 44% starch and 9% fiber (Leonel et al., 2003). Besides, amylose content in isolated turmeric starch was around 48.4% of total starch (Kuttigounder et al., 2011), making the material become swell and burst under the effect of high temperature.
These factors could affect the behavior of the aqueous phase during the thermal process. The interaction between the starch and water under the impact of H+ ion also improves the starch hydrolysis (Moreschi et al., 2006), which could reduce the viscosity of the aqueous phase and increase the diffusion of curcumin during the extraction. Interestingly, turmeric starch starts pasting and gelatinization at temperatures higher than 70 °C (Kuttigounder et al., 2011). So, the OH heating with a temperature lower than 70 °C could not efficiently break the starch granule and release amylose.
In general, the results from OH treatment revealed that pH 5.0 conditions were more effective than pH 2.0 and pH 8.0 conditions (Fig. 2d). Under pH 5.0 conditions, the released curcumin increased by 24% when increase the OH temperature from 50 to 95 °C (Fig. 2d). Figure 4 summarizes the effect of the different pre-treatments and illustrates the opposite effect of US and OH on the particle size distribution of the turmeric powder.
Effects of Pre-treatment on the Color of the Aqueous Extract at Different pH
The color of bioactive compounds is strongly affected by their physical state (the crystalline or the soluble form) (Haas et al., 2019). Also, curcumin’s physical state depends on the pH condition (Lestari & Indrayanto, 2014). Figure 5 shows the differences in curcumin crystals size at different pH conditions. According to Lima et al. (2019), the acidic condition makes polyphenol more stable than neutral/ alkaline conditions, converting polyphenol molecules into carbinol and chalcone pseudo bases. Besides high pH value, degradation of curcumin increases in light, high temperature, and prolonged exposure time (Kiamahalleh et al., 2016; Lestari & Indrayanto, 2014). The curcumin’s degradation under different pH conditions and the effect of pre-treatment on curcumin color were not fully reported in the literature. The preliminary tests show that the extracts’ color varies from light yellow to brown orange in the current study.
In the current experimental setup, the sediment was removed from the extracts by centrifugation before color measurement. The experiment was set up to compare the effect of different pre-treatments and pH conditions on the properties of the extract and curcumin recovery (Table 2). Color changes of the extracts are shown in Fig. 3.
The aqueous extraction is expected to be less selective on curcumin recovery than organic solvent; thus, besides curcumin, extracts contained co-components. Because the main component in turmeric is starch, by more than 40% (Leonel et al., 2003); also, starch dissolved in the aqueous phase at 95 °C, a possible hypothesis is the main co-component in the extract was starch. Thus, the extract’s color could be affected by curcumin content, the physical state of curcumin, the total concentration, and co-components in the extracts. These factors simultaneously impacted the color of the extract; however, the impact factors prevailed at different levels.
Acidic pH Condition (pH 2.0)
At acidic pH conditions, curcumin is insoluble in the aqueous phase (Lestari & Indrayanto, 2014). Notably, released curcumin in acidic conditions was not high as neutral conditions because of the low solubility. Nevertheless, it was found that starch in turmeric was diffused to aqueous phase under acidic conditions. The effect of cation H+ in the aqueous phase ruptures starch granules into the smaller polymers and diffuses to the aqueous phase (Van Beynum & Roels, 1985). A few studies report this phenomenon in turmeric (Gopi et al., 2019; Moreschi et al., 2006; Santana et al., 2017). All of these factors showed the impacts on the color of extracts.
The OH pre-treatment extracts indicate the lowest yellowness (+ b*), shown in Fig. 3b. This result agrees with previous studies and suggests that the thermal effect will reduce the compound’s color (Lafarga et al., 2018; Miglio et al., 2008). In contrast, the lightness of the extracts was not affected by all pre-treatments in the acidic conditions.
Weak Acidic pH Condition (pH 5.0)
In general, in the range of weak acid to neutral pH conditions, curcumin is relatively soluble in the aqueous phase and has an orange-yellow color (Kharat et al., 2017; Lestari et al., 2014). Nevertheless, when curcumin’s solubility increases, it is unstable and quickly degraded by photolytic, high temperature, and oxidation (Kharat et al., 2017; Metzler et al., 2013; Schieffer, 2002). Consequently, the color of the aqueous phase was affected by the amount and physical state of curcumin in the extracts. At pH 5.0, yellowness indicators increased in the extracts with prior pre-treatment. The results showed that extracts with the PEF/HPP had the highest yellowness. In this regard, the reason could be that the curcumin content in these extracts was higher than the extracts with OH and US (Fig. 2a–d).
Weak Alkaline pH Condition (pH 8.0)
Under weak alkaline pH conditions, curcumin is more sensitive and degrades into primary products: ferulic acid and feruloylmethane (Brittain, 2014). These transformations originated from the reaction of a keto-enol group in curcumin molecules (Anand et al., 2008; Niu et al., 2012), and the degradation starts when increasing the pH to neutral conditions (Wang et al., 1997). In the literature, a few studies suggest that the mechanism of the degradation under alkaline pH conditions is the opening of the epoxide (Gordon et al., 2015) and the damage of the conjugated diene structure leading to the destruction of the central heptadienone linkage (Jayaprakasha et al., 2006). By changing the molecule structure, the color of curcumin was also changed to orange-yellow (reduce lightness) in an alkaline aqueous phase (Gordon et al., 2015). In the current study, it can be seen from the figure that all of the extracts at pH 8.0 were darker than in pH 2.0 and 5.0 (Fig. 3). According to Kharat et al. (2017), curcumin remains more than 85% in acidic conditions while remaining lower than 62% in neutral-alkaline conditions. A few studies also agreed that curcumin can be degraded into FA and feruloylmethane, which has a darker color (Gordon et al., 2015; Lestari & Indrayanto, 2014).
The color of curcumin under alkaline pH value is yellow-orange (Lestari & Indrayanto, 2014). This color is unstable and easy to change under weak alkaline pH, resulting in a dramatic fade in yellow-orange color (Gordon et al., 2015; Lestari & Indrayanto, 2014). In summary, under weak alkaline conditions, all extracts with prior pre-treatment have a yellowness reduction compared to the untreated sample (Fig. 3b, d). The yellowness of extracts at pH 8.0 followed the order: Untreated > US > HPP > PEF > OH.
Conclusion
Aqueous extraction of curcumin is challenging due to the low water solubility. Consequently, extraction yields did not surpass 6.6% of the total curcumin in dried turmeric powder. However, pre-treatments and pH variation of the aqueous extraction medium show an effective impact on curcumin recovery. These approaches are essential for the production of curcumin extracts which fall in the legal category of juices and coloring foods. In untreated samples, the extraction at 95 °C shows the highest curcumin recovery after 30 min. Prolonged extraction time reduced the curcumin recovery due to the degradation of the curcumin in the aqueous phase.
Based on the observations with different pre-treatments in this study, it could be stated that acidic pH is the most efficient condition for curcumin recovery, and alkaline pH is not recommended for curcumin extraction. All extracts with and without pre-treatment show high released curcumin levels at pH 2.0 and pH 5.0 compared to 8.0 conditions (except US). The turmeric powder pre-treatment with US shows lower efficiency than other pre-treatments; this is unexpected because US was reported as a fast and effective treatment for polyphenol extraction. However, local thermal effects may have contributed to pronounced degradation of curcumin. The differentiation of mass transfer phenomena that contribute to increased release and degradation, leading to reduced retention of compounds, will require further investigation.
The findings from this research supply deeper understanding about curcuminoid recovery and stability with different aqueous extraction processes. The pre-treatments combined with aqueous extraction meet green extraction and environment-friendliness.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Ade-Omowaye, B., Rastogi, N., Angersbach, A., & Knorr, D. (2001). Effects of high hydrostatic pressure or high intensity electrical field pulse pre-treatment on dehydration characteristics of red paprika. Innovative Food Science Emerging Technologies, 2(1), 1–7.
Aggarwal, S., Ichikawa, H., Takada, Y., Sandur, S. K., Shishodia, S., & Aggarwal, B. B. (2006). Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IκBα kinase and Akt activation. Molecular Pharmacology, 69(1), 195–206.
Ameer, K., Shahbaz, H. M., & Kwon, J. H. (2017). Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Comprehensive Reviews in Food Science and Food Safety, 16(2), 295–315.
Anand, P., Thomas, S. G., Kunnumakkara, A. B., Sundaram, C., Harikumar, K. B., Sung, B., & Rajasekharan, K. N. (2008). Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochemical Pharmacology, 76(11), 1590–1611.
Ando, Y., Mizutani, K., & Wakatsuki, N. (2014). Electrical impedance analysis of potato tissues during drying. Journal of Food Engineering, 121, 24–31.
Angersbach, A., Heinz, V., & Knorr, D. (1999). Electrophysiological model of intact and processed plant tissues: Cell disintegration criteria. Biotechnology Progress, 15(4), 753–762.
Barba, F. J., Zhu, Z., Koubaa, M., Sant’Ana, A. S., & Orlien, V. (2016). Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends in Food Science Technology, 49, 96–109.
Brittain, H. G. (2020). Profiles of drug substances, excipients, and related methodology. Academic press.
Brittain, I. H. G. (2014). Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use.
Butz, P., Koller, W., Tauscher, B., & Wolf, S. (1994). Ultra-high pressure processing of onions: Chemical and sensory changes. LWT-Food Science Technology, 27(5), 463–467.
Chemat, F., Fabiano-Tixier, A. S., Vian, M. A., Allaf, T., & Vorobiev, E. (2015). Solvent-free extraction of food and natural products. TrAC Trends in Analytical Chemistry, 71, 157–168.
Commission, E. P., Medicines, E. D. F. T. Q. O., & Healthcare. (2010). European pharmacopoeia (Vol. 1). Council of Europe.
Donsì, F., Ferrari, G., & Pataro, G. (2010). Applications of pulsed electric field treatments for the enhancement of mass transfer from vegetable tissue. Food Engineering Reviews, 2(2), 109–130.
Fauster, T., Schlossnikl, D., Rath, F., Ostermeier, R., Teufel, F., Toepfl, S., & Jaeger, H. (2018). Impact of pulsed electric field (PEF) pretreatment on process performance of industrial French fries production. Journal of Food Engineering, 235, 16–22.
Flint, E. B., & Suslick, K. S. (1991). The temperature of cavitation. Science, 253(5026), 1397–1399. https://doi.org/10.1126/science.253.5026.1397
Gavahian, M., Sastry, S., Farhoosh, R., & Farahnaky, A. (2020). Ohmic heating as a promising technique for extraction of herbal essential oils: Understanding mechanisms, recent findings, and associated challenges. In Advances in food and nutrition research (Vol. 91, pp. 227–273). Elsevier.
George, J. M., Sowbhagya, H. B., & Rastogi, N. K. (2018). Effect of high pressure pretreatment on drying kinetics and oleoresin extraction from ginger. Drying Technology, 36(9), 1107–1116.
Gopi, S., Amalraj, A., Jude, S., Benson, K., Balakrishnan, P., Haponiuk, J. T., & Thomas, S. (2019). Isolation and characterization of stable nanofiber from turmeric spent using chemical treatment by acid hydrolysis and its potential as antimicrobial and antioxidant activities. Journal of Macromolecular Science, Part A, 56(4), 327–340.
Gordon, O. N., Luis, P. B., Ashley, R. E., Osheroff, N., & Schneider, C. (2015). Oxidative transformation of demethoxy-and bisdemethoxycurcumin: products, mechanism of formation, and poisoning of human topoisomerase IIα. Chemical Research in Toxicology, 28(5), 989–996.
Gulzar, S., & Benjakul, S. (2020). Impact of pulsed electric field pretreatment on yield and quality of lipid extracted from cephalothorax of Pacific white shrimp (Litopenaeus vannamei) by ultrasound-assisted process. International Journal of Food Science Technology, 55(2), 619–630.
Haas, K., Obernberger, J., Zehetner, E., Kiesslich, A., Volkert, M., & Jaeger, H. (2019). Impact of powder particle structure on the oxidation stability and color of encapsulated crystalline and emulsified carotenoids in carrot concentrate powders. Journal of Food Engineering, 263, 398–408.
Hadi, B., Sanagi, M. M., Ibrahim, W. A. W., Jamil, S., AbdullahiMu’azu, M., & Aboul-Enein, H. Y. (2015). Ultrasonic-assisted extraction of curcumin complexed with methyl-β-cyclodextrin. Food Analytical Methods, 8(6), 1373–1381.
Han, Z., Zeng, X. A., Yu, S. J., Zhang, B. S., & Chen, X. D. (2009). Effects of pulsed electric fields (PEF) treatment on physicochemical properties of potato starch. Innovative Food Science Emerging Technologies, 10(4), 481–485.
Huang, H. -W., Hsu, C. -P., Yang, B. B., & Wang, C. -Y. (2013). Advances in the extraction of natural ingredients by high pressure extraction technology. Trends in Food Science Technology, 33(1), 54–62.
Jayaprakasha, G. K., Rao, L. J., & Sakariah, K. K. (2006). Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chemistry, 98(4), 720–724.
Joshi, R. P., Negi, G., Kumar, A., Pawar, Y. B., Munjal, B., Bansal, A. K., & Sharma, S. S. (2013). SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: an insight into its mechanism for neuroprotection. Nanomedicine: Nanotechnology, Biology Medicine, 9(6), 776–785.
Jovanovic, S. V., Steenken, S., Boone, C. W., & Simic, M. G. (1999). H-atom transfer is a preferred antioxidant mechanism of curcumin. Journal of the American Chemical Society, 121(41), 9677–9681.
Kaminaga, Y., Nagatsu, A., Akiyama, T., Sugimoto, N., Yamazaki, T., Maitani, T., & Mizukami, H. (2003). Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Letters, 555(2), 311–316. https://doi.org/10.1016/s0014-5793(03)01265-1
Khan, S. A., Aslam, R., & Makroo, H. A. (2019). High pressure extraction and its application in the extraction of bio-active compounds: A review. Journal of Food Process Engineering, 42(1), e12896.
Kharat, M., Du, Z., Zhang, G., & McClements, D. J. (2017). Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature, and molecular environment. Journal of Agricultural and Food Chemistry, 65(8), 1525–1532.
Kiamahalleh, M. V., Najafpour-Darzi, G., Rahimnejad, M., Moghadamnia, A. A., & Kiamahalleh, M. V. (2016). High performance curcumin subcritical water extraction from turmeric (Curcuma longa L.). Journal of Chromatography B, 1022, 191–198.
Kuttigounder, D., Lingamallu, J. R., & Bhattacharya, S. (2011). Turmeric powder and starch: selected physical, physicochemical, and microstructural properties. Journal of Food Science, 76(9), C1284–C1291.
Lafarga, T., Viñas, I., Bobo, G., Simó, J., & Aguiló-Aguayo, I. (2018). Effect of steaming and sous vide processing on the total phenolic content, vitamin C and antioxidant potential of the genus Brassica. Innovative Food Science Emerging Technologies, 47, 412–420.
Le-Tan, H., Fauster, T., Vladic, J., Gerhardt, T., Haas, K., & Jaeger, H. (2021). Application of Emerging Cell Disintegration Techniques for the Accelerated Recovery of Curcuminoids from Curcuma longa. Applied Sciences, 11(17), 8238. https://www.mdpi.com/2076-3417/11/17/8238. Accessed 25 February 2022.
Lee, K., Chantrasakdakul, P., Kim, D., Kong, M., & Park, K. Y. (2014). Ultrasound pretreatment of filamentous algal biomass for enhanced biogas production. Waste Management, 34(6), 1035–1040. https://doi.org/10.1016/j.wasman.2013.10.012
Leonel, M., Sarmento, S. B., & Cereda, M. P. (2003). New starches for the food industry: Curcuma longa and Curcuma zedoaria. Carbohydrate Polymers, 54(3), 385–388.
Lestari, M. L., & Indrayanto, G. (2014). Curcumin. Profiles of Drug Substances, Excipients and Related Methodology, 39, 113–204. https://doi.org/10.1016/B978-0-12-800173-8.00003-9
Lima, E. M. F., Madalão, M. C. M., dos Santos, W. C., Bernardes, P. C., Saraiva, S. H., & Silva, P. I. (2019). Spray-dried microcapsules of anthocyanin-rich extracts from Euterpe edulis M. as an alternative for maintaining color and bioactive compounds in dairy beverages. Journal of Food Science Technology, 56(9), 4147–4157.
Luengo, E., Martínez, J. M., Álvarez, I., & Raso, J. (2016). Effects of millisecond and microsecond pulsed electric fields on red beet cell disintegration and extraction of betanines. Industrial Crops and Products, 84, 28–33.
Maskooki, A., & Eshtiaghi, M. N. (2012). Impact of pulsed electric field on cell disintegration and mass transfer in sugar beet. Food Bioproducts Processing, 90(3), 377–384.
Metzler, M., Pfeiffer, E., Schulz, S. I., & Dempe, J. S. (2013). Curcumin uptake and metabolism. BioFactors, 39(1), 14–20. https://doi.org/10.1002/biof.1042
Miglio, C., Chiavaro, E., Visconti, A., Fogliano, V., & Pellegrini, N. (2008). Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. Journal of Agriculture and Food Chemistry, 56(1), 139–147. https://doi.org/10.1021/jf072304b
Moreschi, S., Leal, J., Braga, M., & Meireles, M. (2006). Ginger and turmeric starches hydrolysis using subcritical water+ CO2: The effect of the SFE pre-treatment. Brazilian Journal of Chemical Engineering, 23(2), 235–242.
Niu, Y., Ke, D., Yang, Q., Wang, X., Chen, Z., An, X., & Shen, W. (2012). Temperature-dependent stability and DPPH scavenging activity of liposomal curcumin at pH 7.0. Food Chemistry, 135(3), 1377–1382.
Ordóñez-Santos, L. E., Pinzón-Zarate, L. X., & González-Salcedo, L. O. (2015). Optimization of ultrasonic-assisted extraction of total carotenoids from peach palm fruit (Bactris gasipaes) by-products with sunflower oil using response surface methodology. Ultrasonics Sonochemistry, 27, 560–566.
Osorio-Tobón, J. F., Carvalho, P. I., Rostagno, M. A., Petenate, A. J., & Meireles, M. A. A. (2014). Extraction of curcuminoids from deflavored turmeric (Curcuma longa L.) using pressurized liquids: Process integration and economic evaluation. The Journal of Supercritical Fluids, 95, 167–174.
Parniakov, O., Barba, F. J., Grimi, N., Marchal, L., Jubeau, S., Lebovka, N., & Vorobiev, E. (2015). Pulsed electric field and pH assisted selective extraction of intracellular components from microalgae Nannochloropsis. Algal Research, 8, 128–134.
Peng, S., Zou, L., Zhou, W., Liu, W., Liu, C., & McClements, D. J. (2019). Encapsulation of lipophilic polyphenols into nanoliposomes using pH-driven method: Advantages and disadvantages. Journal of Agricultural Food Chemistry, 67(26), 7506–7511.
Pereira, R. N., Coelho, M. I., Genisheva, Z., Fernandes, J. M., Vicente, A. A., & Pintado, M. E. (2020). Using Ohmic Heating effect on grape skins as a pretreatment for anthocyanins extraction. Food Bioproducts Processing, 124, 320–328.
Pereira, R. N., Rodrigues, R. M., Genisheva, Z., Oliveira, H., de Freitas, V., Teixeira, J. A., & Vicente, A. A. (2016). Effects of ohmic heating on extraction of food-grade phytochemicals from colored potato. LWT, 74, 493–503.
Pérez-Elvira, S., Fdz-Polanco, M., Plaza, F., Garralón, G., & Fdz-Polanco, F. (2009). Ultrasound pre-treatment for anaerobic digestion improvement. Water Science Technology, 60(6), 1525–1532.
Pingret, D., Fabiano-Tixier, A.-S., & Chemat, F. (2013). Degradation during application of ultrasound in food processing: A review. Food Control, 31(2), 593–606.
Priyadarsini, K. I. (2014). The chemistry of curcumin: From extraction to therapeutic agent. Molecules, 19(12), 20091–20112. https://doi.org/10.3390/molecules191220091
Rahman, S. M., Telny, T. C., Ravi, T. K., & Kuppusamy, S. (2009). Role of Surfactant and pH in Dissolution of Curcumin. Indian Journal of Pharmaceutical Sciences, 71(2), 139–142. https://doi.org/10.4103/0250-474X.54280
Ramalingam, P., & Ko, Y. T. (2014). A validated LC-MS/MS method for quantitative analysis of curcumin in mouse plasma and brain tissue and its application in pharmacokinetic and brain distribution studies. Journal of Chromatography. b, Analytical Technologies in the Biomedical and Life Sciences, 969, 101–108. https://doi.org/10.1016/j.jchromb.2014.08.009
Santana, Á. L., Zabot, G. L., Osorio-Tobón, J. F., Johner, J. C., Coelho, A. S., Schmiele, M., & Meireles, M. A. A. (2017). Starch recovery from turmeric wastes using supercritical technology. Journal of Food Engineering, 214, 266–276.
Schieffer, G. W. (2002). Pressurized liquid extraction of curcuminoids and curcuminoid degradation products from turmeric (Curcuma longa) with subsequent HPLC assays. Journal of Liquid Chromatography Related Technologies, 25(19), 3033–3044.
Schreider, F. (1968). Technologie des zuckers.
Shirsath, S., Sable, S., Gaikwad, S., Sonawane, S., Saini, D., & Gogate, P. (2017). Intensification of extraction of curcumin from Curcuma amada using ultrasound assisted approach: Effect of different operating parameters. Ultrasonics Sonochemistry, 38, 437–445.
Skrede, G. (1985). Color quality of blackcurrant syrups during storage evaluated by hunter L’, a’, b’values. Journal of Food Science, 50(2), 514–517.
Suslick, K. S., Didenko, Y., Fang, M. M., Hyeon, T., Kolbeck, K. J., McNamara, W. B., III., & Wong, M. (1999). Acoustic cavitation and its chemical consequences. Philosophical Transactions of the Royal Society of London. Series a: Mathematical, Physical Engineering Sciences, 357(1751), 335–353.
Suslick, K. S., Eddingsaas, N. C., Flannigan, D. J., Hopkins, S. D., & Xu, H. (2011). Extreme conditions during multibubble cavitation: Sonoluminescence as a spectroscopic probe. Ultrasonics Sonochemistry, 18(4), 842–846.
Teissie, J., Golzio, M., & Rols, M. (2005). Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of?) knowledge. Biochimica Et Biophysica Acta -General Subjects, 1724(3), 270–280.
Van Beynum, G., & Roels, J. (1985). Starch conversion technology.
Vilkhu, K., Mawson, R., Simons, L., & Bates, D. (2008). Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innovative Food Science & Emerging Technologies, 9(2), 161–169.
Vogel, H., & Pelletier, J. (1815). Curcumin-biological and medicinal properties. Journal of Pharmaceutics, 2(50), 24.
Vorobiev, E., & Lebovka, N. (2006). Extraction of intercellular components by pulsed electric fields. In Pulsed electric fields technology for the food industry (pp. 153–193). Springer.
Wang, Y. J., Pan, M. H., Cheng, A. L., Lin, L. I., Ho, Y. S., Hsieh, C. Y., & Lin, J. K. (1997). Stability of curcumin in buffer solutions and characterization of its degradation products. Journal of Pharmaceutical and Biomedical Analysis, 15(12), 1867–1876. https://doi.org/10.1016/s0731-7085(96)02024-9
Yu, X., Gouyo, T., Grimi, N., Bals, O., & Vorobiev, E. (2016). Pulsed electric field pretreatment of rapeseed green biomass (stems) to enhance pressing and extractives recovery. Bioresource Technology, 199, 194–201. https://doi.org/10.1016/j.biortech.2015.08.073
Yucel, U., Alpas, H., & Bayindirli, A. (2010). Evaluation of high pressure pretreatment for enhancing the drying rates of carrot, apple, and green bean. Journal of Food Engineering, 98(2), 266–272.
Yusoff, M. M., Gordon, M. H., Ezeh, O., & Niranjan, K. (2017). High pressure pre-treatment of Moringa oleifera seed kernels prior to aqueous enzymatic oil extraction. Innovative Food Science Emerging Technologies, 39, 129–136.
Zheng, B., Zhang, X., Peng, S., & Julian McClements, D. (2019). Impact of curcumin delivery system format on bioaccessibility: Nanocrystals, nanoemulsion droplets, and natural oil bodies. Food & Function, 10(7), 4339–4349. https://doi.org/10.1039/c8fo02510j
Acknowledgements
The authors gratefully acknowledge the EQ-BOKU VIBT GmbH and the Food Stability Center as well as the BOKU Core Facility Food & Bio Processing for providing the experimental facilities used in this work. The authors thank GNT-Europa GmbH (Germany) for providing the turmeric powder used for trials. In addition, the authors would like to thank Brian Gallogly and Mary Violet Berger for their support during the experimental setup.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). This study was supported by the University of Natural Resources and Life Sciences Vienna (BOKU) in the frame of the OeAD Grant No. Ref. MPC-2020–01134.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le-Tan, H., Fauster, T., Haas, K. et al. Aqueous Extraction of Curcuminoids from Curcuma longa: Effect of Cell Disintegration Pre-treatment and Extraction Condition. Food Bioprocess Technol 15, 1359–1373 (2022). https://doi.org/10.1007/s11947-022-02820-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-022-02820-5