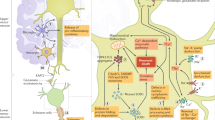
Abstract
Purpose of Review
While amyotrophic lateral sclerosis (ALS) remains a progressive fatal diagnosis, there have been numerous advances in the last several years, both in disease-modifying and symptomatic treatment. This review aims to provide a comprehensive and updated review of the literature of current treatment options for ALS.
Recent Findings
We will discuss the proposed mechanisms of action, evidence for efficacy, and safety profiles for the four current Food and Drug Administration (FDA)–approved disease-modifying treatments: riluzole, edaravone, AMX0035 (combination of sodium phenylbutyrate and taurursodiol) and tofersen. Additionally, we will review several therapies that are under active investigation for the more common genetic forms of ALS. Finally, we will discuss options for symptomatic treatment, including a review of Nuedexta (combination of dextromethorphan hydrobromide and quinidine sulfate) approved for pseudobulbar affect, but recent evidence is suggesting that it also improves bulbar function.
Summary
There are four FDA-approved disease-modifying treatments for ALS, which likely confer a modest benefit in survival with good safety profile and tolerability, through different mechanisms of action. More post-marketing and population studies will be needed to assess the overall efficacy of each medication and potential combinations of each.
Similar content being viewed by others
References and Recommended Reading
Masrori P, Van Damme P. Amyotrophic lateral sclerosis: a clinical review. Eur J Neurol. 2020;27(10):1918–29.
Stetkarova I, Ehler E. Diagnostics of amyotrophic lateral sclerosis: up to date. Diagnostics (Basel). 2021;11(2).
Kiernan MC, et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat Rev Neurol. 2021;17(2):104–18.
Dorst J, Ludolph AC, Huebers A. Disease-modifying and symptomatic treatment of amyotrophic lateral sclerosis. Ther Adv Neurol Disord. 2018;11:1756285617734734.
Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev. 2012;2012(3):CD001447.
Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med, 1994;330(9):585–91.
Lacomblez L, et al. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347(9013):1425–31.
Bensimon G, et al. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. J Neurol. 2002;249(5):609–15.
Yanagisawa N, et al. Efficacy and safety of riluzole in patients with amyotrophic lateral sclerosis: double-blind placebo-controlled study in Japan. Igakuno Ayumi. 1997;182(11):851–66.
Andrews JA, et al. Real-world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(7–8):509–18.
Lacomblez L, et al. Long-term safety of riluzole in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002;3(1):23–9.
Cruz MP. Edaravone (Radicava): a novel neuroprotective agent for the treatment of amyotrophic lateral sclerosis. P t. 2018;43(1):25–8.
Abe K, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(7–8):610–7.
Edaravone Als 16 Study, G., A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(sup1):11–19.
Tanaka M, et al. A 24-week, phase III, double-blind, parallel-group study of Edaravone (MCI-186) for treatment of amyotrophic lateral sclerosis (ALS) (P3.189). Neurology. 2016;86(16 Supplement): p. P3.189.
Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16(7):505–512.
Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS severity classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(sup1):40–48.
Witzel S, et al. Safety and effectiveness of long-term intravenous administration of edaravone for treatment of patients with amyotrophic lateral sclerosis. JAMA Neurol. 2022;79(2):121–30.
Ishizaki K, et al. Real-world safety of the novel, free radical scavenger edaravone for amyotrophic lateral sclerosis patients: data from the post-marketing surveillance SUNRISE Japan. Neurol Clin Neurosci. 2021;9(3):223–9.
Paganoni S, et al. Long-term survival of participants in the CENTAUR trial of sodium phenylbutyrate-taurursodiol in amyotrophic lateral sclerosis. Muscle Nerve. 2021;63(1):31–9.
Paganoni S, et al. Trial of sodium phenylbutyrate–taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919–30.
Paganoni S, et al. Survival analyses from the CENTAUR trial in amyotrophic lateral sclerosis: evaluating the impact of treatment crossover on outcomes. Muscle Nerve. 2022;66(2):136–41.
Inc AP. Phase III trial of AMX0035 for amyotrophic lateral sclerosis treatment. 2021. https://ClinicalTrials.gov/show/NCT05021536.
Van Mossevelde S, et al. Relationship between C9orf72 repeat size and clinical phenotype. Curr Opin Genet Dev. 2017;44:117–24.
AI Therapeutics I. Study of safety, tolerability, and biological activity of LAM-002A in C9ORF72-associated amyotrophic lateral sclerosis. 2021. https://ClinicalTrials.gov/show/NCT05163886.
Inc A. A phase 2 study to evaluate AL001 in C9orf72-associated ALS. 2021. https://ClinicalTrials.gov/show/NCT05053035.
Ltd., W.L.S., Study of WVE-004 in patients with C9orf72-associated amyotrophic lateral sclerosis (ALS) or frontotemporal dementia (FTD). 2021. https://ClinicalTrials.gov/show/NCT04931862.
Florida UO. Safety and therapeutic potential of the FDA-approved drug metformin for C9orf72 ALS/FTD. 2020. https://ClinicalTrials.gov/show/NCT04220021.
Biogen, A study to assess the safety, tolerability, and pharmacokinetics of BIIB078 in adults with C9ORF72-associated amyotrophic lateral sclerosis. 2018. https://ClinicalTrials.gov/show/NCT03626012.
Transposon Therapeutics I. A phase 2a study of TPN-101 in patients with C9ORF72 ALS/FTD. 2021. https://ClinicalTrials.gov/show/NCT04993755.
Peggion C, et al. SOD1 in ALS: taking stock in pathogenic mechanisms and the role of glial and muscle cells. Antioxidants (Basel). 2022;11(4).
Miller TM, et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099–110.
Biogen, A Study of BIIB067 When Initiated in Clinically Presymptomatic Adults With a Confirmed Superoxide Dismutase 1 Mutation. 2021. https://ClinicalTrials.gov/show/NCT04856982.
Nolan M, Talbot K, Ansorge O. Pathogenesis of FUS-associated ALS and FTD: insights from rodent models. Acta Neuropathol Commun. 2016;4(1):99.
Korobeynikov VA, et al. Antisense oligonucleotide silencing of FUS expression as a therapeutic approach in amyotrophic lateral sclerosis. Nat Med. 2022;28(1):104–16.
Ionis Pharmaceuticals I. A study to evaluate the efficacy, safety, pharmacokinetics and pharmacodynamics of ION363 in amyotrophic lateral sclerosis participants with fused in sarcoma mutations (FUS-ALS). 2021. https://ClinicalTrials.gov/show/NCT04768972.
Elden AC, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466(7310):1069–75.
van den Heuvel DM, et al. Taking a risk: a therapeutic focus on ataxin-2 in amyotrophic lateral sclerosis? Trends Mol Med. 2014;20(1):25–35.
Biogen. A study to assess the safety, tolerability, and effect on disease progression of BIIB105 in participants with amyotrophic lateral sclerosis (ALS) and participants with the ALS Ataxin-2 (ATXN2) Genetic Mutation (ALSpire). 2020.
Pinto AC, et al. Respiratory assistance with a non-invasive ventilator (Bipap) in MND/ALS patients: survival rates in a controlled trial. J Neurol Sci. 1995;129(Suppl):19–26.
Kleopa KA, et al. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci. 1999;164(1):82–8.
Bourke SC, et al. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5(2):140–7.
Vitacca M, et al. Impact of an early respiratory care programme with non-invasive ventilation adaptation in patients with amyotrophic lateral sclerosis. Eur J Neurol. 2018;25(3):556-e33.
Dorst J, Ludolph AC. Non-invasive ventilation in amyotrophic lateral sclerosis. Ther Adv Neurol Disord. 2019;12:1756286419857040.
Weiss MD, et al. A randomized trial of mexiletine in ALS: safety and effects on muscle cramps and progression. Neurology. 2016;86(16):1474–81.
Cruz MP. Nuedexta for the treatment of pseudobulbar affect: a condition of involuntary crying or laughing. P T. 2013;38(6):325–8.
Panitch HS, et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann Neurol. 2006;59(5):780–7.
Brooks BR, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology. 2004;63(8):1364–70.
Pioro EP, et al. Dextromethorphan plus ultra low-dose quinidine reduces pseudobulbar affect. Ann Neurol. 2010;68(5):693–702.
Smith R, et al. Enhanced bulbar function in amyotrophic lateral sclerosis: the nuedexta treatment trial. Neurotherapeutics. 2017;14(3):762–72.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Author Senda Ajroud-Driss has served on advisory boards for Biogen, Amylyx and Orphazyme in the past two years. Author Shubadra Priyadarshini has no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Priyadarshini, S., Ajroud-Driss, S. Update on ALS Treatment. Curr Treat Options Neurol 25, 199–212 (2023). https://doi.org/10.1007/s11940-023-00757-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-023-00757-4