Abstract
Purpose of review
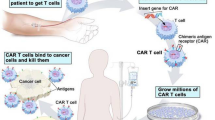
Chimeric antigen receptor T cell (CAR-T) adoptive cell therapy is an effective treatment for patients with refractory B cell malignancies. As its use has grown, there has been an increase in the incidence of a serious, potentially fatal neurotoxicity known as immune effector cell-associated neurotoxicity syndrome (ICANS). This review discusses the clinical manifestations of this neurotoxicity syndrome, current grading systems, management strategies, and proposed biologic mechanisms leading to neurotoxicity.
Recent findings
Current research suggests that patients with a higher disease burden and higher CAR-T cell doses are positively associated with the development of ICANS, as are elevated serum levels of proinflammatory cytokines and the presence of cytokine release syndrome (CRS). While patterns observed on neuroimaging and electroencephalogram (EEG) are non-specific for the diagnosis of ICANS, each modality may provide helpful clinical information such as the detection of cerebral edema, the most serious of associated symptoms. Anti-epileptic medications and corticosteroids may ameliorate the symptoms of ICANS.
Summary
The mechanism for ICANS is currently unknown; however, systemic inflammation and cytokine production triggering a cascade of endothelial activation and BBB disruption likely contribute. With limited treatment options available, further clinical research into the precise mechanism and treatment is urgently needed as the use of CAR-T and other adoptive cell therapies continues to grow.

Similar content being viewed by others
Change history
09 August 2019
The correct spelling of the co-author should be listed as Sarah Nagle, MD.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48. This was the pivotal clinical trial leading to the FDA approval of tisagenlecleucel.
• Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44. This was the pivotal clinical trial leading to the FDA approval of axicabtagene.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59.
Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–38.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet Lond Engl. 2015;385:517–28.
Kochenderfer JN, Dudley ME, Kassim SH, Somerville RPT, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33:540–9.
Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42.
YESCARTA [package insert]. Santa Monica: Kite Pharma; 2017.
•• Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8:958–71. This paper is one of the largest observational studies characterizing the clinical syndrome of CAR-T neurotoxicity.
• Gust J, Hay KA, Hanafi L-A, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–19. This paper provides the foundational basic science evidence for the proposed mechanism of CAR-T neurotoxicity.
Research AA for C. JCAR015 in ALL: a root-cause investigation. Cancer Discov. 2018;8:4–5.
Turtle CJ, Riddell SR, Maloney DG. CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy for B-cell malignancies. Clin Pharmacol Ther. 2016;100:252–8.
Chang ZL, Chen YY. CARs: Synthetic immunoreceptors for cancer therapy and beyond. Trends Mol Med. 2017;23:430–50.
Hay KA, Hanafi L-A, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–306.
Titov A, Petukhov A, Staliarova A, Motorin D, Bulatov E, Shuvalov O, et al. The biological basis and clinical symptoms of CAR-T therapy-associated toxicites. Cell Death Dis [Internet]. 2018 [cited 2018 Dec 21];9. Available from: http://www.nature.com/articles/s41419-018-0918-x.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24:20–8.
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant [Internet] 2018 [cited 2019 Mar 31]; Available from: https://linkinghub.elsevier.com/retrieve/pii/S1083879118316914.
•• Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2017;15:47–62. This paper provides key recommendations regarding the management on neurologic toxicities due to CAR-T cell therapy and proposed and early clinical grading system, the CARTOX 10.
Britton J, Frey L, Hopp J. The abnormal EEG. Electroencephalogr EEG Introd Text Atlas Norm Abnorm Find Adults Child Infants Internet [Internet]. Chicago: American Epilepsy Society; 2016. Available from: https://www.ncbi.nlm.nih.gov/books/NBK390357/
Herlopian A, Dietrich J, Abramson JS, Cole AJ, Westover MB. EEG findings in CAR T cell therapy-related encephalopathy. Neurology. 2018;91:227–9.
Locke FL, Neelapu SS, Bartlett NL, Lekakis LJ, Jacobson CA, Braunschweig I, et al. Preliminary results of prophylactic tocilizumab after axicabtagene ciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL). Blood. 2017;130:1547.
Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–30.
Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-targeted CAR-T cell therapies. CNS Drugs. 2018;32:1091–101.
Teachey DT, Bishop MR, Maloney DG, Grupp SA. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit “ALL”. Nat Rev Clin Oncol. 2018;15:218.
Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J Immunol. 1993;151:4081–9.
Gardner R, Leger KJ, Annesley CE, Summers C, Rivers J, Gust J, et al. Decreased rates of severe CRS seen with early intervention strategies for CD19 CAR-T cell toxicity management. Blood. 2016;128:586.
Grevich S, Shenoi S. Update on the management of systemic juvenile idiopathic arthritis and role of IL-1 and IL-6 inhibition. Adolesc Health Med Ther. 2017;8:125–35.
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–48.
Mackall CL, Miklos DB. CNS endothelial cell activation emerges as a driver of CAR T cell–associated neurotoxicity. Cancer Discov. 2017;7:1371–3.
Wardill HR, Mander KA, Van Sebille YZA, Gibson RJ, Logan RM, Bowen JM, et al. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int J Cancer. 2016;139:2635–45.
Taraseviciute A, Tkachev V, Ponce R, Turtle CJ, Snyder JM, Liggitt HD, et al. Chimeric antigen receptor T cell–mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8:750–63.
Mueller KT, Maude SL, Porter DL, Frey N, Wood P, Han X, et al. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130:2317–25.
Ramos CA, Ballard B, Zhang H, Dakhova O, Gee AP, Mei Z, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. 2017;127:3462–71.
Wang C-M, Wu Z-Q, Wang Y, Guo Y-L, Dai H-R, Wang X-H, et al. Autologous T cells expressing CD30 chimeric antigen receptors for relapsed or refractory Hodgkin lymphoma: an open-label phase I trial. Clin Cancer Res. 2017;23:1156–66.
Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S–15S.
Clark IA, Vissel B. Excess cerebral TNF causing glutamate excitotoxicity rationalizes treatment of neurodegenerative diseases and neurogenic pain by anti-TNF agents. J Neuroinflammation. 2016;13:236.
Pemberton LA, Kerr SJ, Smythe G, Brew BJ. Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J Interf Cytokine Res. 1997;17:589–95.
Croitoru-Lamoury J, Guillemin GJ, Dormont D, Brew BJ. Quinolinic acid up-regulates chemokine production and chemokine receptor expression in astrocytes. Adv Exp Med Biol. 2003;527:37–45.
Tavares RG, Tasca CI, Santos CES, Alves LB, Porciúncula LO, Emanuelli T, et al. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem Int. 2002;40:621–7.
Baranyi A, Amouzadeh-Ghadikolai O, von Lewinski D, Breitenecker RJ, Stojakovic T, März W, et al. Beta-trace protein as a new non-invasive immunological marker for quinolinic acid-induced impaired blood-brain barrier integrity. Sci Rep. 2017;7:43642.
Tavares RG, Schmidt AP, Tasca CI, Souza DO. Quinolinic acid-induced seizures stimulate glutamate uptake into synaptic vesicles from rat brain: effects prevented by guanine-based purines. Neurochem Res. 2008;33:97–102.
Severino PC, Muller G do AS, Vandresen-Filho S, Tasca CI. Cell signaling in NMDA preconditioning and neuroprotection in convulsions induced by quinolinic acid. Life Sci. 2011;89:570–6.
Eid T, Gruenbaum SE, Dhaher R, Lee T-SW, Zhou Y, Danbolt NC. The glutamate-glutamine cycle in epilepsy. Adv Neurobiol. 2016;13:351–400.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Holly E. Hinson has consulted for Biogen on an ischemic stroke study unrelated to the current work. All other authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The correct spelling of the co-author should be listed as Sarah Nagle, MD.
This article is part of the Topical Collection on Critical Care Neurology
Rights and permissions
About this article
Cite this article
Rice, J., Nagle, S., Randall, J. et al. Chimeric Antigen Receptor T Cell-Related Neurotoxicity: Mechanisms, Clinical Presentation, and Approach to Treatment. Curr Treat Options Neurol 21, 40 (2019). https://doi.org/10.1007/s11940-019-0580-3
Published:
DOI: https://doi.org/10.1007/s11940-019-0580-3