Opinion statement
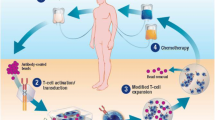
Chimeric antigen receptor (CAR) T-cells are now a well-established treatment for hematologic malignancies. Their use in clinical practice has expanded quite rapidly and hospitals have developed CAR T-cell protocols to evaluate patients for associated toxicities, and particularly for neurotoxicity. There are many variables that influence the risk for developing this complication, many of which are not fully understood. The severity can be related to a particular product. Clinical vigilance is critical to facilitate early recognition of neurotoxicity, hence the importance of pre-CAR T-cell neurological evaluation of each patient. While details of such an evaluation may slightly differ between institutions, generally a comprehensive neurological evaluation including assessment of cognitive abilities along with magnetic resonance imaging (MRI) of the brain is a gold standard. Management of neurotoxicity requires a well-orchestrated team approach with specialists from oncology, neurology, oftentimes neurosurgery and neuro-intensive care. Diagnostic work-up frequently includes detailed neurologic evaluation with comparison to the baseline assessment, imaging of the brain, electroencephalogram, and lumbar puncture. While steroids are uniformly used for treatment, many patients also receive tocilizumab for an underlying and frequently concomitant cytokine release syndrome (CRS) in addition to symptom-driven supportive care. Novel CAR T-cell constructs and other agents allowing for potentially lower risk of toxicity are being explored. While neurotoxicity is predominantly an early, and reversible, event, a growing body of literature suggests that late neurotoxicity with variable clinical presentation can also occur.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
• Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol. 2020;17(3):147–67 This review comprehensively discusses chimeric antigen receptor design and engineering strategies being developed to overcome current challenges.
Denlinger N, Bond D, Jaglowski S. CAR T-cell therapy for B-cell lymphoma. Curr Probl Cancer. 2022;46(1):100826.
Benmebarek MR, et al. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci. 2019;20(6):1283.
Maude SL, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48.
Schuster SJ, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56.
Neelapu SS, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44.
Abramson JS, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52.
Wang M, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–42.
Munshi NC, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–16.
Berdeja JG, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–24.
Martin T, et al. Ciltacabtagene autoleucel, an anti-B-cell maturation antigen chimeric antigen receptor T-cell therapy, for relapsed/refractory multiple myeloma: CARTITUDE-1 2-year follow-up. J Clin Oncol. 2022. https://doi.org/10.1200/JCO.22.00842.
Fischer JW, Bhattarai N. CAR-T cell therapy: mechanism, management, and mitigation of inflammatory toxicities. Front Immunol. 2021;12:693016.
van der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14(7):499–509.
Neelapu SS, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62.
Park JH, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59.
Schuster SJ, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–54.
Weinkove R, et al. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunology. 2019;8(5):e1049.
Grigor EJM, et al. Risks and benefits of chimeric antigen receptor T-cell (CAR-T) therapy in cancer: a systematic review and meta-analysis. Transfus Med Rev. 2019;33(2):98–110.
Morris EC, et al. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol. 2022;22(2):85–96.
Hay KA, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130(21):2295–306.
Santomasso BD, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958–71.
Sterner RM, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133(7):697–709.
Kang L, et al. Interleukin-6-knockdown of chimeric antigen receptor-modified T cells significantly reduces IL-6 release from monocytes. Exp Hematol Oncol. 2020;9:11.
Gust J, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–19.
Torre M, et al. Neuropathology of a case with fatal CAR T-cell-associated cerebral edema. J Neuropathol Exp Neurol. 2018;77(10):877–82.
Turtle CJ, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010–20.
Grant SJ, et al. Clinical presentation, risk factors, and outcomes of immune effector cell-associated neurotoxicity syndrome following chimeric antigen receptor T cell therapy: a systematic review. Transplant Cell Ther. 2022;28(6):294–302.
Karschnia P, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–21.
Rubin DB, et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain. 2019;142(5):1334–48.
Tallantyre EC, et al. Neurological updates: neurological complications of CAR-T therapy. J Neurol. 2021;268(4):1544–54.
•• Maus MV, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events. J Immunother Cancer. 2020;8(2):e001511 The most recent Society for Immunotherapy of Cancer clinical practice guideline on immune effector cell-related adverse events comprehensively outlines an evidence- and consensus- based approach to cellular therapy-related toxicity.
Hayden PJ, et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann Oncol. 2022;33(3):259–75.
Thompson JA, et al. Management of immunotherapy-related toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(4):387–405.
Lee DW, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38.
Chakraborty R, et al. Late effects after chimeric antigen receptor T cell therapy for lymphoid malignancies. Transplant Cell Ther. 2021;27(3):222–9.
Gonzalez Castro LN, Dietrich J. Evaluation and management of chimeric antigen receptor (CAR) T-cell-associated neurotoxicity. Neurooncol Pract. 2021;8(3):259–65.
Santomasso BD, et al. Management of immune-related adverse events in patients treated with chimeric antigen receptor T-cell therapy: ASCO Guideline. J Clin Oncol. 2021;39(35):3978–92.
Locke FL, et al. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory,aggressive non-Hodgkin lymphoma (NHL). Blood. 2017;130:1547.
• Scholler N, et al. Tumor immune contexture is a determinant of anti-CD19 CAR T cell efficacy in large B cell lymphoma. Nat Med. 2022;28(9):1872–82 This paper examined pre-treatment and post-treatment tumor immune contexture and its influence on CAR T-cell-related outcomes among patients on the ZUMA-1 trial. They found that a pre-treatment tumor microenvironment with a low density of regulatory T-cells was associated with an increased risk of neurotoxicity in those treated with axicabtagene ciloleucel.
• Saini NY, et al. Clonal hematopoiesis is associated with increased risk of severe neurotoxicity in axicabtagene ciloleucel therapy of large B-cell lymphoma. Blood Cancer Discov. 2022;3(5):385–93 This publication examined the impact of clonal hematopoiesis prior to receiving axicabtagene ciloleucel on outcomes and found it was associated with an increased risk of severe neurotoxicity.
Gaulin C, Kelemen K, Arana Yi C. Molecular pathways in clonal hematopoiesis: from the acquisition of somatic mutations to transformation into hematologic neoplasm. Life. 2022;12(8):1135.
Novo M, et al. Axicabtagene ciloleucel chimeric antigen receptor T cell therapy in lymphoma with secondary central nervous system involvement. Mayo Clin Proc. 2019;94(11):2361–4.
Frigault MJ, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–6.
Lin Y, et al. Experience with axicabtagene ciloleucel (Axi-cel) in patients with secondary CNS involvement: results from the US Lymphoma CAR T Consortium. Blood. 2019;134(Supplement_1):763.
Ghafouri S, et al. Axicabtagene Ciloleucel CAR T-cell therapy for relapsed/refractory secondary CNS non-Hodgkin lymphoma: comparable outcomes and toxicities, but shorter remissions may warrant alternative consolidative strategies? Bone Marrow Transplant. 2021;56(4):974–7.
Abramson JS, et al. Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. N Engl J Med. 2017;377(8):783–4.
Traube C, et al. Cornell Assessment of Pediatric Delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med. 2014;42(3):656–63.
Castaneda-Puglianini O, Chavez JC. Assessing and management of neurotoxicity after CAR-T therapy in diffuse large B-cell lymphoma. J Blood Med. 2021;12:775–83.
Locke FL, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898–911.
Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019;111(7):646–54.
Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021;56(3):552–66.
Rubin DB, et al. Clinical predictors of neurotoxicity after chimeric antigen receptor T-cell therapy. JAMA Neurol. 2020;77(12):1536–42.
Nellan A, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018;132(6):662–6.
•• Oluwole OO, et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br J Haematol. 2021;194(4):690–700 This analysis of an exploratory cohort from the ZUMA-1 trial found that prophylactic and early corticosteroids and/or tocilizumab resulted in a low rate of neurologic events in patients who received axicabtagene ciloleucel without compromising efficacy.
Jatiani SS, et al. Myeloma CAR-T CRS management with IL-1R antagonist anakinra. Clin Lymphoma Myeloma Leuk. 2020;20(9):632–636 e1.
Wehrli M, et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J Immunother Cancer. 2022;10(1):01.
Strati P, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–7.
Shah NN, et al. Intrathecal chemotherapy for management of steroid-refractory CAR T-cell-associated neurotoxicity syndrome. Blood Adv. 2020;4(10):2119–22.
Garfall AL, et al. Posterior reversible encephalopathy syndrome (PRES) after infusion of anti-Bcma CAR T cells (CART-BCMA) for multiple myeloma: successful treatment with cyclophosphamide. Blood. 2016;128(22):5702.
Kenderian SS, et al. ZUMA-19: a phase 1/2 multicenter study of lenzilumab use with axicabtagene ciloleucel (axi-cel) in patients (pts) with relapsed or refractory large B cell lymphoma (R/R LBCL). Blood. 2020;136(Supplement 1):6–7.
Zhang L, et al. Etanercept as a new therapeutic option for cytokine release syndrome following chimeric antigen receptor T cell therapy. Exp Hematol Oncol. 2021;10(1):16.
Kenderian SS, et al. Ruxolitinib prevents cytokine release syndrome after CART cell therapy without impairing the anti-tumor effect in a xenograft model. Blood. 2016;128(22):652.
Pan J, et al. Ruxolitinib mitigates steroid-refractory CRS during CAR T therapy. J Cell Mol Med. 2021;25(2):1089–99.
Weber EW, et al. Pharmacologic control of CAR-T cell function using dasatinib. Blood Adv. 2019;3(5):711–7.
Ruella M, et al. The addition of the BTK inhibitor ibrutinib to anti-CD19 chimeric antigen receptor T cells (CART19) improves responses against mantle cell lymphoma. Clin Cancer Res. 2016;22(11):2684–96.
Ruella M, et al. Kinase inhibitor ibrutinib to prevent cytokine-release syndrome after anti-CD19 chimeric antigen receptor T cells for B-cell neoplasms. Leukemia. 2017;31(1):246–8.
Siegler EL, Kenderian SS. Neurotoxicity and cytokine release syndrome after chimeric antigen receptor T cell therapy: insights into mechanisms and novel therapies. Front Immunol. 2020;11:1973.
Maillet D, et al. Evaluation of mid-term (6-12 months) neurotoxicity in B-cell lymphoma patients treated with CAR T cells: a prospective cohort study. Neuro Oncol. 2021;23(9):1569–75.
Ursu R, et al. Long-term neurological safety in B-cell lymphoma patients treated with anti-CD19 CAR T-cell therapy. Neurology 2022.
Cordeiro A, et al. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26(1):26–33.
Ruark J, et al. Patient-reported neuropsychiatric outcomes of long-term survivors after chimeric antigen receptor T cell therapy. Biol Blood Marrow Transplant. 2020;26(1):34–43.
Hoogland AI, et al. Change in neurocognitive performance among patients with non-Hodgkin lymphoma in the first year after chimeric antigen receptor T cell therapy. Transplant Cell Ther. 2022;28(6):305 e1–9.
Van Oekelen O, et al. Neurocognitive and hypokinetic movement disorder with features of parkinsonism after BCMA-targeting CAR-T cell therapy. Nat Med. 2021;27(12):2099–103.
•• Cohen AD, et al. Incidence and management of CAR-T neurotoxicity in patients with multiple myeloma treated with ciltacabtagene autoleucel in CARTITUDE studies. Blood Cancer J. 2022;12(2):32 This publication outlines the strategies implemented in patients receiving ciltacabtagene autoleucel in the CARTITUDE trials to monitor for and manage parkinsonism, among other movement and neurocognitive treatment-emergent adverse events.
ABECMA® (idecabtagene vicleucel), suspension for intravenous infusion F.a.D. Administration, Editor. 2021, Celgene Corporation, a Bristol-Myers Squibb Company: Summit, NJ.
Schmitt M, et al. Third-generation CAR T cells targeting CD19 are associated with an excellent safety profile and might improve persistence of CAR T cells in treated patients. Blood. 2019;134(Supplement_1):51.
Roselli E, Faramand R, Davila ML. Insight into next-generation CAR therapeutics: designing CAR T cells to improve clinical outcomes. J Clin Invest. 2021;131(2):e142030.
Spiegel JY, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–31.
Shah NN, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med. 2020;26(10):1569–75.
Depil S, et al. 'Off-the-shelf' allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–99.
Laskowski TJ, Biederstadt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22:557–75.
Bishop MR, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386(7):629–39.
Locke FL, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–54.
Usmani SZ, et al. KarMMa-4: Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T-cell therapy in high-risk newly diagnosed multiple myeloma. Journal of Clinical Oncology. 2021;39(15_suppl):TPS8053-TPS8053.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Fortin Ensign has nothing to disclose. Dr. Gaulin has nothing to disclose. Dr. Hrachova has nothing to disclose. Dr. Ruff has nothing to disclose. Dr. Harahsheh has nothing to disclose. Dr. Vicenti has nothing to disclose. Dr. Castro has nothing to disclose. Dr. Munoz reports personal fees from Pharmacyclics/Abbvie, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, BMS, Kyowa, Alexion, Fosunkite, Innovent, Seattle Genetics, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, Beigene, Servier, Novartis, Morphosys/Incyte, Mei pharma, Zodiac, grants from Bayer, Gilead/Kite Pharma, Celgene, Merck, Portola, Incyte, Genentech, Pharmacyclics, Seattle Genetics, Janssen, Millennium, other from Targeted Oncology, OncView, Curio, Kyowa, Physicians’ Education Resource, Dava, Global clinical insights, MJH, Shanghai Youyao, and Seattle Genetics, other from Gilead/Kite Pharma, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, Beigene, Verastem, AstraZeneca, Celgene/BMS, Genentech/Roche, during the conduct of the study. Dr. Rosenthal has nothing to disclose. Dr. Mrugala has nothing to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fortin Ensign, S.P., Gaulin, C., Hrachova, M. et al. Evaluating the Patient with Neurotoxicity after Chimeric Antigen Receptor T-cell Therapy. Curr. Treat. Options in Oncol. 23, 1845–1860 (2022). https://doi.org/10.1007/s11864-022-01035-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-01035-2