Abstract
Purpose of Review
Conventional and novel applications of Poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors (PARPi) are reviewed in the context of recently published clinical trials and preclinical data supporting rapidly expanding uses of this class of chemotherapy.
Recent Findings
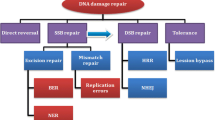
PARPi block a pathway of DNA repair and target defects in homologous recombination repair (HRR), a pathway responsible for high-fidelity repair of double-strand breaks in DNA. BRCA1/2 proteins are essential to this pathway. Approximately 15–30% of women with ovarian cancer will have a germline or somatic BRCA mutation, and PARPi have shown promise in this population in a variety of settings. With growing understanding of the HRR pathway and its role in gynecologic malignancies, the potential applications of PARPi continue to expand.
Summary
While the role of PARPi in gynecologic malignancies is most established in ovarian cancer, there are also promising applications in uterine and cervical cancer. We review current indications for PARPi use and promising applications of these medications in gynecologic malignancies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481:287–94.
Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. https://doi.org/10.1038/nature03445.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. https://doi.org/10.1038/nature03443.
Gadducci A, Guarneri V, Peccatori FA, Ronzino G, Scandurra G, Zamagni C, et al. Current strategies for the targeted treatment of high-grade serous epithelial ovarian cancer and relevance of BRCA mutational status. J Ovarian Res. Journal of Ovarian Research. 2019;12:9–8. https://doi.org/10.1186/s13048-019-0484-6.
Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. https://doi.org/10.1016/j.bpobgyn.2016.08.006.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin Wiley-Blackwell. 2011;61:69–90.
National Cancer Institute: Surveillance, Epidemiology and ERP. Cancer stat facts: ovarian cancer. Natl Cancer Inst [Internet]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: ovarian cancer including fallopian tube cancer and primary peritoneal cancer. Natl Compr Cancer Netw. 2019;Version 2. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med [Internet]. 2012;366:1382–92. https://doi.org/10.1177/1758835919849753.
Food and Drug Administration. Label: Lynparza [Internet]. 2016 [cited 2019 Jul 21]. Available from: www.fda.gov/medwatch.
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol [Internet].Lancet Publishing Group. 2017;18:1274–84. https://doi.org/10.1016/S1470-2045(17)30469-2.
Mirza MR, Åvall Lundqvist E, Birrer MJ, de Pont Christensen R, Nyvang G-B, Malander S, et al. Niraparib plus bevacizumab versus niraparib alone for platinum-sensitive recurrent ovarian cancer (NSGO-AVANOVA2/ENGOT-ov24): a randomised, phase 2, superiority trial. Lancet Oncol Elsevier BV. 2019;20:1409–19.
Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:1949–61. https://doi.org/10.1016/S0140-6736(17)32440-6.
•• Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495–505. https://doi.org/10.1056/NEJMoa1810858The results of this phase 3 randomized control trial led to the first approval of PARP inhibitor maintenance after first-line chemotherapy for women with aBRCAmutation and ovarian, fallopian tube or primary peritoneal cancer.
• González-Martín A, Pothuri B, Vergote I, DePont CR, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391–402. https://doi.org/10.1056/NEJMoa1910962This is the first study of niraparib in frontline chemotherapy, and likely to lead to approval for its use as maintenance in the upfront setting.
• Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N Engl J Med. 2019;381:2403–15. https://doi.org/10.1056/NEJMoa1909707This is the first study of a PARP inhibitor concurrent with chemotherapy. However, because no arm compared chemotherapy alone followed by PARP maintenance to veliparib throughout, the benefit of adding veliparib to chemotherapy prior to PARP maintenance is yet to be determined.
•• Ray-Coquard IL, Pautier P, Pignata S, et al. Phase III PAOLA-1/ENGOT-ov25 trial: olaparib plus bevacizumab (bev) as maintenance therapy in patients (pts) with newly diagnosed, advanced ovarian cancer (OC) treated with platinum-based chemotherapy (PCh) plus bev. Eur Soc Med Oncol. 2019; This is the only study evaluating the combination of a PARP inhibitor with bevacizumab in the front-line setting. Because no arm compared PARP inhibitor maintenance to PARP/bevacizumab maintenance, the additional impact of bevacizumab to PARP maintenance, especially in the in patients with HRD or BRCA mutations, has not been established.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol Proc Am Soc Clin Oncol. 2015;33:244–50.
Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372–9.
Swisher EM, Lin KK, Oza AM, Scott CL, Giordano H, Sun J, et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): an international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. https://doi.org/10.1016/S1470-2045(16)30559-9.
Dal Molin GZ, Omatsu K, Sood AK, Coleman RL. Rucaparib in ovarian cancer: an update on safety, efficacy and place in therapy. Ther Adv Med Oncol. 2018. https://doi.org/10.1177/1758835918778483.
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol Elsevier Ltd. 2011;12:852–61. https://doi.org/10.1016/S1470-2045(11)70214-5.
Vanderstichele A, Van Nieuwenhuysen E, Han S, Concin N, Van Gorp T, Berteloot P, et al. Randomized phase II CLIO study on olaparib monotherapy versus chemotherapy in platinum-resistant ovarian cancer. J Clin Oncol American Society of Clinical Oncology. 2019;37:5507. https://doi.org/10.1200/JCO.2019.37.15_suppl.5507.
Food and Drug Administration. FDA approves niraparib for HRD-positive advanced ovarian cancer [Internet]. 10/23/2019. 2019 [cited 2019 Jan 11]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-hrd-positive-advanced-ovarian-cancer
• Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R, et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol.; 2019;5:1141–1149. This is one of the first trials to evaluate the combination of a PARP inhibitor and immunotherapy in ovarian cancer. While final results are still pending, the initial results were promising among a heavily pretreated population and indicate a potential role for this combination in ovarian cancer.
• Drew Y, de Jonge M, Hong SH, Park YH, Wolfer A, Brown J, et al. An open-label, phase II basket study of olaparib and durvalumab (MEDIOLA): results in germline BRCA -mutated ( gBRCA m) platinum-sensitive relapsed (PSR) ovarian cancer (OC). Gynecol Oncol. 2018;149:246–7 This is one of the first studies looking at immunotherapy combined with PARP inhibition. Data are still very early; however initial results show a response rate of approximately 63%.
Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol Lancet Publishing Group. 2014;15:1207–14.
Mirza MR, Avall-Lundqvist E, Birrer MJ, Christensen R, de Pont NG-B, Malander S, et al. Combination of niraparib and bevacizumab versus niraparib alone as treatment of recurrent platinum-sensitive ovarian cancer: a randomized controlled chemotherapy-free study—NSGO-AVANOVA2/ENGOT-OV24. J Clin Oncol. American Society of Clinical Oncology;. 2019;37:5505. https://doi.org/10.1200/JCO.2019.37.15_suppl.5505.
Rehman FL, Lord CJ, Ashworth A. The promise of combining inhibition of PI3K and PARP as cancer therapy. Cancer Discov. 2012;2:982–4.
Westin SN, Litton JK, Williams RA, Shepherd CJ, Brugger W, Pease EJ, et al. Phase I trial of olaparib (PARP inhibitor) and vistusertib (mTORC1/2 inhibitor) in recurrent endometrial, ovarian and triple negative breast cancer. J Clin Oncol. American Society of Clinical Oncology. 2018;36:5504. https://doi.org/10.1200/JCO.2018.36.15_suppl.5504.
Matulonis UA, Wulf GM, Barry WT, Birrer M, Westin SN, Farooq S, et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann Oncol. 2017;28:512–8.
Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. Elsevier Ltd. 2019;20:570–80. https://doi.org/10.1016/S1470-2045(18)30905-7.
National Cancer Institute: Surveillance, Epidemiology and ERP. Cancer stat facts: uterine cancer [Internet]. [cited 2019 Jun 10]. Available from: https://seer.cancer.gov/statfacts/html/corp.html
Lheureux S, McCourt C, Rimel BJ, Duska L, Fleming G, Mackay H, et al. Moving forward with actionable therapeutic targets and opportunities in endometrial cancer: a NCI clinical trials planning meeting report. Gynecol Oncol Elsevier Inc. 2018;149:442–6. https://doi.org/10.1016/j.ygyno.2018.02.005.
Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–7.
Słopień R, Męczekalski B. Aromatase inhibitors in the treatment of endometriosis. Menopausal Rev. 2016;1:43–7. https://doi.org/10.5114/pm.2016.58773.
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–108 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673615001300.
Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2:24–6.
Bian X, Gao J, Luo F, Rui C, Zheng T, Wang D, et al. PTEN deficiency sensitizes endometrioid endometrial cancer to compound PARP-PI3K inhibition but not PARP inhibition as monotherapy. Oncogene. Nature Publishing Group. 2018;37:341–51.
Forster MD, Dedes KJ, Sandhu S, Frentzas S, Kristeleit R, Ashworth A, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol. 2011;8:302–6.
Philip CA, Laskov I, Beauchamp MC, Marques M, Amin O, Bitharas J, et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer BMC Cancer. 2017;17:1–11.
Mak JPY, Ma HT, Poon RYC. Synergism between ATM and PARP1 inhibition involves DNA damage and abrogating the G 2 DNA damage checkpoint. 2019;
Mitamura T, Dong P, Ihira K, Kudo M, Watari H. Molecular-targeted therapies and precision medicine for endometrial cancer. Jpn J Clin Oncol. 2019;49:108–20.
Aghajanian C, Sill MW, Secord AA, Powell MA, Steinhoff M. Iniparib plus paclitaxel and carboplatin as initial treatment of advanced or recurrent uterine carcinosarcoma: a gynecologic oncology group study. Gynecol Oncol. Elsevier Inc. 2012;126:424–7. https://doi.org/10.1016/j.ygyno.2012.05.024.
Hensley ML, Barrette BA, Baumann K, Gaffney D, Hamilton AL, Kim JW, et al. Gynecologic Cancer InterGroup (GCIG) consensus review: uterine and ovarian leiomyosarcomas. Int J Gynecol Cancer. Lippincott Williams and Wilkins; 2014. p. S61–6.
Roberts ME, Aynardi JT, Chu CS. Uterine leiomyosarcoma: a review of the literature and update on management options. Gynecol. Oncol. Academic Press Inc.; 2018. p. 562–72.
• Seligson ND, Kautto EA, Passen EN, Stets C, Toland AE, Millis SZ, et al. BRCA1/2 functional loss defines a targetable subset in leiomyosarcoma. Oncologist. 2019;24:973–9. https://doi.org/10.1634/theoncologist.2018-0448This study demonstrated that a subset of leiomyosarcomas possess BRCA mutations, and establishes the potential role for PARP inhibitors in uterine sarcoma.
Tewari KS, Monk BJ. Gynecologic oncology group trials of chemotherapy for metastatic and recurrent cervical cancer. Curr Oncol Rep. 2005;7:419–34.
Boussios S, Seraj E, Zarkavelis G, Petrakis D, Kollas A, Kafantari A, et al. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: where do we stand? A literature review. Crit Rev Oncol Hematol. Elsevier Ireland Ltd. 2016:164–74.
Prasad CB, Prasad SB, Yadav SS, Pandey LK, Singh S, Pradhan S, et al. Olaparib modulates DNA repair efficiency, sensitizes cervical cancer cells to cisplatin and exhibits anti-metastatic property. Sci Rep. Springer US. 2017;7:1–15. https://doi.org/10.1038/s41598-017-13232-3.
Bianchi A, Lopez S, Altwerger G, Bellone S, Bonazzoli E, Zammataro L, et al. PARP-1 activity (PAR) determines the sensitivity of cervical cancer to olaparib. Gynecol Oncol [Internet]. Elsevier Inc.; 2019;4–10. https://doi.org/10.1016/j.ygyno.2019.08.010.
Mann M, Sharma A, Kumar S, Chauhan SS, Bhatla N, Kumar S, et al. PARP-1 inhibitor modulate β-catenin signaling to enhance cisplatin sensitivity in cancer cervix. Oncotarget. 2019;10:4262–75.
Chatterjee P, Choudhary GS, Sharma A, Singh K, Heston WD, Ciezki J, et al. PARP inhibition sensitizes to low dose-rate radiation TMPRSS2-ERG fusion gene-expressing and PTEN-deficient prostate cancer cells. PLoS One. 2013;8:1–12.
Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–43.
Chung HC, Ros W, Delord J-P, Perets R, Italiano A, Shapira-Frommer R, et al. Efficacy and safety of pembrolizumab in previously treated advanced cervical cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2019;37:1470–8. https://doi.org/10.1200/JCO.18.01265.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Gynecologic Cancers
Rights and permissions
About this article
Cite this article
Lightfoot, M., Montemorano, L. & Bixel, K. PARP Inhibitors in Gynecologic Cancers: What Is the Next Big Development?. Curr Oncol Rep 22, 29 (2020). https://doi.org/10.1007/s11912-020-0873-4
Published:
DOI: https://doi.org/10.1007/s11912-020-0873-4