Abstract
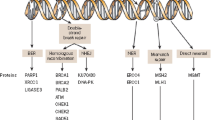
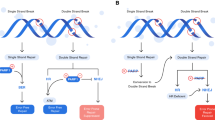
Ovarian cancer has the highest mortality rate among all gynecologic cancers. The standard treatment for over 40 years has been surgery and chemotherapy; however, over the last decade, targeted therapies are increasingly proven effective. Poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi) are molecules that competitively block one of the DNA repair pathways by binding to PARP, and thus push the repair process to the homologous recombination pathway. In patients with a BRCA1/2 mutation, this pathway is deficient and ultimately leads to apoptosis. Patients who harbor a BRCA1/2 mutation are at higher risk of developing certain cancers such as breast and ovarian, rendering prophylactic strategies attractive in this subset of patients. PARPi have been found to be beneficial in this group of patients; recently, various international regulatory agencies have approved this class of agents as maintenance therapy or as monotherapy in the relapsed setting. Contemporary trials are ongoing in the relapsed as well as first-line setting, monotherapy and maintenance therapy or even in combination with other agents. This review focuses on the mechanisms involved in DNA repair pertinent to BRCA1/2 mutation and PAPR inhibitor therapy, the prophylactic and therapeutic advances in this field, and the opportunities available.
Similar content being viewed by others
References
Online Analysis. [cited 2017 Apr 27]. http://globocan.iarc.fr/Pages/online.aspx.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
SEER Cancer Statistics Review. Previous version—SEER cancer statistics; 1975–2007. [cited 2017 May 3]. https://seer.cancer.gov/archive/csr/1975_2007/.
Meric-Bernstam F, Farhangfar C, Mendelsohn J, Mills GB. Building a personalized medicine infrastructure at a major cancer center. J Clin Oncol. 2013;31(15):1849–57.
Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411(6835):366–74.
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15.
Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21.
Prevalence and penetrance of. BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Anglian Breast Cancer Study Group. Br J Cancer. 2000;83(10):1301–8.
Whittemore AS, Gong G, John EM, McGuire V, Li FP, Ostrow KL, et al. Prevalence of BRCA1 mutation carriers among U.S. non-Hispanic Whites. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2078–83.
King M-C, Marks JH, Mandell JB, New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–6.
Petrucelli N, Daly MB, Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. 1998 Sep 4 [Updated 2016 Dec 15]. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJ, et al., editors. GeneReviews(®). Seattle: University of Washington; 1993 [cited 2017 Apr 8]. http://www.ncbi.nlm.nih.gov/books/NBK1247/.
Kim H, Choi DH. Distribution of BRCA1 and BRCA2 Mutations in Asian Patients with Breast Cancer. J Breast Cancer. 2013;16(4):357–65.
Guo F, Hirth JM, Lin Y-L, Richardson G, Levine L, Berenson AB, et al. Use of BRCA mutation test in the US, 2004–2014. Am J Prev Med. 2017;52:702–9.
Desai S, Jena AB. Do celebrity endorsements matter? Observational study of BRCA gene testing and mastectomy rates after Angelina Jolie’s New York Times editorial. BMJ. 2016;14(355):i6357.
Li X, You R, Wang X, Liu C, Xu Z, Zhou J, et al. Effectiveness of prophylactic surgeries in BRCA1 or BRCA2 mutation carriers: a meta-analysis and systematic review. Clin Cancer Res Off J Am Assoc Cancer Res. 2016;22(15):3971–81.
Karakasis K, Burnier JV, Bowering V, Oza AM, Lheureux S. Ovarian cancer and BRCA1/2 testing: opportunities to improve clinical care and disease prevention. Front Oncol. 2016; 6 [cited 2017 May 6]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862980/.
Przybycin CG, Kurman RJ, Ronnett BM, Shih I-M, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34(10):1407–16.
Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol. 2013;209(5):409–14.
Committee on Gynecologic Practice. Committee opinion no. 620: salpingectomy for ovarian cancer prevention. Obstet Gynecol. 2015;125(1):279–81.
Tschernichovsky R, Goodman A. Risk-reducing strategies for ovarian cancer in BRCA mutation carriers: a balancing act. Oncologist. 2017;22(4):450–9.
King MC, Wieand S, Hale K, Lee M, Walsh T, Owens K, et al. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286(18):2251–6.
Narod SA, Brunet JS, Ghadirian P, Robson M, Heimdal K, Neuhausen SL, et al. Tamoxifen and risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: a case-control study. Hereditary Breast Cancer Clinical Study Group. Lancet Lond Engl. 2000;356(9245):1876–81.
Amé J-C, Spenlehauer C, de Murcia G. The PARP superfamily. BioEssays News Rev Mol Cell Dev Biol. 2004;26(8):882–93.
O’Sullivan Coyne G, Chen AP, Meehan R, Doroshow JH. PARP inhibitors in reproductive system cancers: current use and developments. Drugs. 2017;77(2):113–30.
Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011;5(4):387–93.
Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–20.
Turner N, Tutt A, Ashworth A. Hallmarks of “BRCAness” in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–9.
Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–7.
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28(15):2512–9.
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–34.
Mateo J, Friedlander M, Sessa C, Leunen K, Nicum S, Gourley C, et al. Administration of continuous/intermittent olaparib in ovarian cancer patients with a germline BRCA1/2 mutation to determine an optimal dosing schedule for the tablet formulation. Eur J Cancer. 2013;49:S161–S161
Ricks TK, Chiu H-J, Ison G, Kim G, McKee AE, Kluetz P, et al. Successes and challenges of PARP inhibitors in cancer therapy. Front Oncol. 2015;5 [cited 2017 May 7]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4604313/.
Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RHJ, Sonke GS, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16(1):87–97.
Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50.
Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–84.
SGO 48th Annual Meeting on Women’s Cancer®. [cited 2017 May 7]. https://sgo.confex.com/sgo/2017/meetingapp.cgi/Paper/8718.
Shen Y, Aoyagi-Scharber M, Wang B. Trapping poly(ADP-Ribose) polymerase. J Pharmacol Exp Ther. 2015;353(3):446–57.
Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–64.
Rucaparib_RKristeleit_ESMO2016.pdf. [cited 2017 May 7]. http://clovisoncology.com/files/Rucaparib_RKristeleit_ESMO2016.pdf.
Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. 2017;0(0) [cited 2017 Sep 20]. http://thelancet.com/journals/lancet/article/PIIS0140-6736(17)32440-6/abstract.
Montoni A, Robu M, Pouliot É, Shah GM. Resistance to PARP-inhibitors in cancer therapy. Front Pharmacol. 2013;27:4.
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AOH, Zander SAL, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105(44):17079–84.
Kondrashova O, Nguyen M, Shield-Artin K, Tinker AV, Teng NNH, Harrell MI, et al. Secondary somatic mutations restoring RAD51C and RAD51D associated with acquired resistance to the PARP inhibitor rucaparib in high-grade ovarian carcinoma. Cancer Discov. 2017;7(9):984–98.
Jaspers JE, Kersbergen A, Boon U, Sol W, van Deemter L, Zander SA, et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013;3(1):68–81.
Liu X, Han EK, Anderson M, Shi Y, Semizarov D, Wang G, et al. Acquired resistance to combination treatment with temozolomide and ABT-888 is mediated by both base excision repair and homologous recombination DNA repair pathways. MCR. 2009;7(10):1686–92.
Alotaibi M, Sharma K, Saleh T, Povirk LF, Hendrickson EA, Gewirtz DA. Radiosensitization by PARP inhibition in DNA repair proficient and deficient tumor cells: proliferative recovery in senescent cells. Radiat Res. 2016;185(3):229–45.
Liu JF, Barry WT, Birrer M, Lee J-M, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15(11):1207–14.
Oplustilova L, Wolanin K, Mistrik M, Korinkova G, Simkova D, Bouchal J, et al. Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP-1 inhibitor treatment. Cell Cycle. 2012;11(20):3837–50.
Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–51.
Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61.
Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol Off J Am Soc Clin Oncol. 2015;33(3):244–50.
Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4):372–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cyriac, S.L., Oza, A.M. & Karakasis, K. DNA Repair Defects for Therapy in Ovarian Cancer: The BRCA1/2 and PARP Inhibitor Story. Indian J Gynecol Oncolog 15 (Suppl 1), 65–75 (2017). https://doi.org/10.1007/s40944-017-0155-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40944-017-0155-8