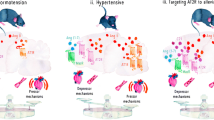
Abstract
Purpose of Review
To review recent data that suggest opposing effects of brain angiotensin type-1 (AT1R) and type-2 (AT2R) receptors on blood pressure (BP). Here, we discuss recent studies that suggest pro-hypertensive and pro-inflammatory actions of AT1R and anti-hypertensive and anti-inflammatory actions of AT2R. Further, we propose mechanisms for the interplay between brain angiotensin receptors and neuroinflammation in hypertension.
Recent Findings
The renin-angiotensin system (RAS) plays an important role in regulating cardiovascular physiology. This includes brain AT1R and AT2R, both of which are expressed in or adjacent to brain regions that control BP. Activation of AT1R within those brain regions mediate increases in BP and cause neuroinflammation, which augments the BP increase in hypertension. The fact that AT1R and AT2R have opposing actions on BP suggests that AT1R and AT2R may have similar opposing actions on neuroinflammation. However, the mechanisms by which brain AT1R and AT2R mediate neuroinflammatory responses remain unclear.
Summary
The interplay between brain angiotensin receptor subtypes and neuroinflammation exacerbates or protects against hypertension.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Dzau VJ. Circulating versus local renin-angiotensin system in cardiovascular homeostasis. Circulation. 1988;77(6 Pt 2):I4–13.
Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology. 2009;89(4):370–6. https://doi.org/10.1159/000211202.
de Kloet AD, Steckelings UM, Sumners C. Protective angiotensin type 2 receptors in the brain and hypertension. Curr Hypertens Rep. 2017;19(6):46. https://doi.org/10.1007/s11906-017-0746-x.
Braga VA, Medeiros IA, Ribeiro TP, Franca-Silva MS, Botelho-Ono MS, Guimaraes DD. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: implications in neurogenic hypertension. Braz J Med Biol Res. 2011;44(9):871–6. https://doi.org/10.1590/s0100-879x2011007500088.
Nunes FC, Braga VA. Chronic angiotensin II infusion modulates angiotensin II type I receptor expression in the subfornical organ and the rostral ventrolateral medulla in hypertensive rats. J Renin-Angiotensin-Aldosterone Syst. 2011;12(4):440–5. https://doi.org/10.1177/1470320310394891.
Zhu G-Q, Patel KP, Zucker IH, Wang W. Microinjection of ANG II into paraventricular nucleus enhances cardiac sympathetic afferent reflex in rats. Am J Phys Heart Circ Phys. 2002;282(6):H2039–H45. https://doi.org/10.1152/ajpheart.00854.2001.
de Kloet AD, Wang L, Pitra S, Hiller H, Smith JA, Tan Y, et al. A unique “angiotensin-sensitive” neuronal population coordinates neuroendocrine, cardiovascular, and behavioral responses to stress. J Neurosci. 2017;37(13):3478–90. https://doi.org/10.1523/JNEUROSCI.3674-16.2017This paper demonstrated cardiovascular-related actions mediated by AT1R located on a specific set of neurons.
Oliveira-Sales EB, Toward MA, Campos RR, Paton JF. Revealing the role of the autonomic nervous system in the development and maintenance of Goldblatt hypertension in rats. Auton Neurosci. 2014;183(100):23–9. https://doi.org/10.1016/j.autneu.2014.02.001.
Oliveira-Sales EB, Colombari E, Abdala AP, Campos RR, Paton JF. Sympathetic overactivity occurs before hypertension in the two-kidney, one-clip model. Exp Physiol. 2016;101(1):67–80. https://doi.org/10.1113/EP085390.
Unger T, Steckelings UM, dos Santos RAS. The protective arm of the renin angiotensin system (RAS): functional aspects and therapeutic implications: Academic Press; 2015.
De Kloet AD, Pitra S, Wang L, Hiller H, Pioquinto DJ, Smith JA, et al. Angiotensin type-2 receptors influence the activity of vasopressin neurons in the paraventricular nucleus of the hypothalamus in male mice. Endocrinology. 2016;157(8):3167–80. https://doi.org/10.1210/en.2016-1131This paper has provided the first documentation of cardiovascular-related actions (secretion of AVP) mediated by AT2R located on a specific set of neurons.
McCarthy CA, Widdop RE, Denton KM, Jones ES. Update on the angiotensin AT 2 receptor. Curr Hypertens Rep. 2013;15(1):25–30. https://doi.org/10.1007/s11906-012-0321-4.
Brouwers S, Smolders I, Wainford RD, Dupont AG. Hypotensive and sympathoinhibitory responses to selective central AT2 receptor stimulation in spontaneously hypertensive rats. Clin Sci. 2015;129(1):81–92. https://doi.org/10.1042/CS20140776.
Dai S-Y, Peng W, Zhang Y-P, Li J-D, Shen Y, Sun X-F. Brain endogenous angiotensin II receptor type 2 (AT2-R) protects against DOCA/salt-induced hypertension in female rats. J Neuroinflammation. 2015;12(1):47. https://doi.org/10.1186/s12974-015-0261-4This paper is the first to demonstrate anti-inflammatory actions mediated by AT2R in DOCA-salt hypertensive rats.
Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens. 2011;24(6):724–30. https://doi.org/10.1038/ajh.2011.33.
Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension. 2008;51(2):521–7. https://doi.org/10.1161/HYPERTENSIONAHA.107.101717.
Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Front Neuroendocrinol. 1997;18(4):383–439. https://doi.org/10.1006/frne.1997.0155.
Millan MA, Jacobowitz DM, Aguilera G, Catt KJ. Differential distribution of AT1 and AT2 angiotensin II receptor subtypes in the rat brain during development. Proc Natl Acad Sci. 1991;88(24):11440–4. https://doi.org/10.1073/pnas.88.24.11440.
de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, et al. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct. 2016;221(2):891–912. https://doi.org/10.1007/s00429-014-0943-1This paper reports the development of a transgenic AT2R reporter mouse, which has enabled the discrete cellular localization of AT2R within and near brain cardiovascular control centers.
Sumners C, Alleyne A, Rodríguez V, Pioquinto DJ, Ludin JA, Kar S, et al. Brain angiotensin type-1 and type-2 receptors: cellular locations under normal and hypertensive conditions. Hypertens Res. 2020;43(4):281–95. https://doi.org/10.1038/s41440-019-0374-8This paper reports the development of a dual transgenic AT1R/AT2R reporter mouse, which has enabled the discrete cellular localization of AT1R and AT2R within and near the brain cardiovascular control centers.
Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, et al. Microglia participate in neurogenic regulation of hypertension. Hypertension. 2015;66(2):309–16. https://doi.org/10.1161/HYPERTENSIONAHA.115.05333.
Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, et al. Brain microglial cytokines in neurogenic hypertension. Hypertension. 2010;56(2):297–303. https://doi.org/10.1161/HYPERTENSIONAHA.110.150409.
Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, et al. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol. 2011;203(2):289–97. https://doi.org/10.1111/j.1748-1716.2011.02313.xThis paper demonstrated pro-inflammatory actions mediated by AT1R in the cardiovascular control centers of the brain.
Sharma RK, Yang T, Oliveira AC, Lobaton GO, Aquino V, Kim S, et al. Microglial cells impact gut microbiota and gut pathology in angiotensin II-induced hypertension. Circ Res. 2019;124(5):727–36. https://doi.org/10.1161/CIRCRESAHA.118.313882.
Korim WS, Elsaafien K, Basser JR, Setiadi A, May CN, Yao ST. In renovascular hypertension, TNF-α type-1 receptors in the area postrema mediate increases in cardiac and renal sympathetic nerve activity and blood pressure. Cardiovasc Res. 2018;115(6):1092–101. https://doi.org/10.1093/cvr/cvy268.
Elsaafien K, Korim WS, Setiadi A, May CN, Yao ST. Chemoattraction and recruitment of activated immune cells, central autonomic control and blood pressure regulation. Front Physiol. 2019;10:984. https://doi.org/10.3389/fphys.2019.00984.
Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res. 2015;117(2):178–91. https://doi.org/10.1161/CIRCRESAHA.117.305853.
Ahmari N, Santisteban MM, Miller DR, Geis NM, Larkin RM, Redler TL, et al. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. Am J Phys Heart Circ Phys. 2019;317(2):H279–H89. https://doi.org/10.1152/ajpheart.00510.2018.
Zubcevic J, Santisteban MM, Perez PD, Arocha R, Hiller H, Malphurs WL, et al. A single angiotensin II hypertensive stimulus is associated with prolonged neuronal and immune system activation in Wistar-Kyoto rats. Front Physiol. 2017;8:592. https://doi.org/10.3389/fphys.2017.00592.
Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood–brain barrier. Hypertension. 2013;63(3):572–9. https://doi.org/10.1161/HYPERTENSIONAHA.113.01743.
Biancardi VC, Stranahan AM, Krause EG, de Kloet AD, Stern JE. Cross talk between AT1 receptors and Toll-like receptor 4 in microglia contributes to angiotensin II-derived ROS production in the hypothalamic paraventricular nucleus. Am J Phys Heart Circ Phys. 2016;310(3):H404–H15. https://doi.org/10.1152/ajpheart.00247.2015.
McCarthy CA, Vinh A, Miller AA, Hallberg A, Alterman M, Callaway JK, et al. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PLoS One. 2014;9(4):e95762. https://doi.org/10.1371/journal.pone.0095762.
Jackson L, Dong G, Althomali W, Sayed MA, Eldahshan W, Baban B, et al. Delayed administration of angiotensin II type 2 receptor (AT2R) agonist compound 21 prevents the development of post-stroke cognitive impairment in diabetes through the modulation of microglia polarization. Transl Stroke Res. 2019:1–14. https://doi.org/10.1007/s12975-019-00752-5.
de Kloet AD, Pioquinto DJ, Nguyen D, Wang L, Smith JA, Hiller H, et al. Obesity induces neuroinflammation mediated by altered expression of the renin–angiotensin system in mouse forebrain nuclei. Physiol Behav. 2014;136:31–8. https://doi.org/10.1016/j.physbeh.2014.01.016.
Tigerstedt R, Bergman P. Niere und Kreislauf 1. Skandinavisches Archiv Für Physiologie. 1898;8(1):223–71. https://doi.org/10.1111/j.1748-1716.1898.tb00272.x.
Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am J Cardiol. 2002;89(2):3–9. https://doi.org/10.1016/s0002-9149(01)02321-9.
Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2011;4(3):1201–28. https://doi.org/10.1002/cphy.c130040.
Chung O, Stoll M, Unger T. Physiologic and pharmacologic implications of AT1 versus AT2 receptors. Blood Press Suppl. 1996;2:47–52.
De Gasparo M, Catt K, Inagami T, Wright J, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52(3):415–72.
Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, et al. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci. 1995;92(8):3521–5. https://doi.org/10.1073/pnas.92.8.3521.
Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, et al. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19(5):464–74. https://doi.org/10.1161/01.hyp.19.5.464.
Koprdová R, Cebová M, Kristek F. Long-term effect of losartan administration on blood pressure, heart and structure of coronary artery of young spontaneously hypertensive rats. Physiol Res. 2009;58(3):327–35.
Park JB, Intengan HD, Schiffrin EL. Reduction of resistance artery stiffness by treatment with the AT1-receptor antagonist losartan in essential hypertension. J Renin-Angiotensin-Aldosterone Syst. 2000;1(1):40–5. https://doi.org/10.3317/jraas.2000.009.
Rodrigo E, Maeso R, Muñoz-García R, Navarro-Cid J, Ruilope LM, Cachofeiro V, et al. Endothelial dysfunction in spontaneously hypertensive rats: consequences of chronic treatment with losartan or captopril. J Hypertens. 1997;15(6):613–8. https://doi.org/10.1097/00004872-199715060-00007.
Assersen K, Sumners C, Steckelings UM. The renin-angiotensin system in hypertension, a constantly renewing classic: focus on the angiotensin AT2-receptor. Can J Cardiol. 2020;36(5):683–93. https://doi.org/10.1016/j.cjca.2020.02.095.
Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377(6551):748–50. https://doi.org/10.1038/377748a0.
Gross V, Milia AF, Plehm R, Inagami T, Luft FC. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J Hypertens. 2000;18(7):955–61. https://doi.org/10.1097/00004872-200018070-00018.
Arima S, Endo Y, Yaoita H, Omata K, Ogawa S, Tsunoda K, et al. Possible role of P-450 metabolite of arachidonic acid in vasodilator mechanism of angiotensin II type 2 receptor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1997;100(11):2816–23. https://doi.org/10.1172/JCI119829.
Endo Y, Arima S, Yaoita H, Omata K, Tsunoda K, Takeuchi K, et al. Function of angiotensin II type 2 receptor in the postglomerular efferent arteriole. Kidney Int Suppl. 1997;63:S205–S7.
Endo Y, Arima S, Yaoita H, Tsunoda K, Omata K, Ito S. Vasodilation mediated by angiotensin II type 2 receptor is impaired in afferent arterioles of young spontaneously hypertensive rats. J Vasc Res. 1998;35(6):421–7. https://doi.org/10.1159/000025613.
Carey RM, Howell NL, Jin X-H, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38(6):1272–7. https://doi.org/10.1161/hy1201.096576.
Li XC, Widdop RE. AT2 receptor-mediated vasodilatation is unmasked by AT1 receptor blockade in conscious SHR. Br J Pharmacol. 2004;142(5):821–30. https://doi.org/10.1038/sj.bjp.0705838.
Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. Response to letter regarding article, “AT2Receptor Activation Induces Natriuresis and Lowers Blood Pressure”. Circ Res. 2014;115(9):e26–e7. https://doi.org/10.1161/CIRCRESAHA.114.304975.
Kemp BA, Howell NL, Keller SR, Gildea JJ, Padia SH, Carey RM. AT2 receptor activation prevents sodium retention and reduces blood pressure in angiotensin II–dependent hypertension. Circ Res. 2016;119(4):532–43. https://doi.org/10.1161/CIRCRESAHA.116.308384.
Schramm LP, Strack AM, Platt KB, Loewy AD. Peripheral and central pathways regulating the kidney: a study using pseudorabies virus. Brain Res. 1993;616(1–2):251–62. https://doi.org/10.1016/0006-8993(93)90216-a.
Weindl A. Neuroendocrine aspects of circumventricular organs. Front Neuroendocrinol. 1973;3:3–32.
Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801(1–2):239–43. https://doi.org/10.1016/s0006-8993(98)00587-3.
Blessing W, Hedger S, Joh T, Willoughby J. Neurons in the area postrema are the only catecholamine-synthesizing cells in the medulla or pons with projections to the rostral ventrolateral medulla (C 1-area) in the rabbit. Brain Res. 1987;419(1):336–40. https://doi.org/10.1016/0006-8993(87)90604-4.
Dampney RA, Czachurski J, Dembowsky K, Goodchild AK, Seller H. Afferent connections and spinal projections of the pressor region in the rostral ventrolateral medulla of the cat. J Auton Nerv Syst. 1987;20(1):73–86. https://doi.org/10.1016/0165-1838(87)90083-x.
Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20(9):1675–88. https://doi.org/10.1097/00004872-200209000-00002.
Sá JM, Barbosa RM, Menani JV, De Luca Jr LA, Colombari E, Colombari DSA. Cardiovascular and hidroelectrolytic changes in rats fed with high-fat diet. Behav Brain Res. 2019;373:112075. https://doi.org/10.1016/j.bbr.2019.112075.
Papas S, Smith P, Ferguson AV. Electrophysiological evidence that systemic angiotensin influences rat area postrema neurons. Am J Phys Regul Integr Comp Phys. 1990;258(1):R70–R6. https://doi.org/10.1152/ajpregu.1990.258.1.R70.
Lowes VL, McLean LE, Kasting NW, Ferguson AV. Cardiovascular consequences of microinjection of vasopressin and angiotensin II in the area postrema. Am J Phys Regul Integr Comp Phys. 1993;265(3):R625–R31. https://doi.org/10.1152/ajpregu.1993.265.3.R625.
Carter DA, Choong Y-T, Connelly AA, Bassi JK, Hunter NO, Thongsepee N, et al. Functional and neurochemical characterization of angiotensin type 1A receptor-expressing neurons in the nucleus of the solitary tract of the mouse. Am J Phys Regul Integr Comp Phys. 2017;313(4):R438–R49. https://doi.org/10.1152/ajpregu.00168.2017.
Chen D, Jancovski N, Bassi JK, Nguyen-Huu T-P, Choong Y-T, Palma-Rigo K, et al. Angiotensin type 1A receptors in C1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress. J Neurosci. 2012;32(6):2051–61. https://doi.org/10.1523/JNEUROSCI.5360-11.2012.
Gonzalez AD, Wang G, Waters EM, Gonzales KL, Speth RC, Van Kempen TA, et al. Distribution of angiotensin type 1a receptor-containing cells in the brains of bacterial artificial chromosome transgenic mice. Neuroscience. 2012;226:489–509. https://doi.org/10.1016/j.neuroscience.2012.08.039.
Hasser EM, Cunningham JT, Sullivan MJ, Curtis KS, Blaine EH, Hay M. Area postrema and sympathetic nervous system effects of vasopressin and angiotensin II. Clin Exp Pharmacol Physiol. 2000;27(5–6):432–6. https://doi.org/10.1046/j.1440-1681.2000.03261.x.
Nahey DB, Collister JP. ANG II-induced hypertension and the role of the area postrema during normal and increased dietary salt. Am J Phys Heart Circ Phys. 2007;292(1):H694–700. https://doi.org/10.1152/ajpheart.00998.2005.
Chao J, Gao J, Parbhu K-JK, Gao L. Angiotensin type 2 receptors in the intermediolateral cell column of the spinal cord: negative regulation of sympathetic nerve activity and blood pressure. Int J Cardiol. 2013;168(4):4046–55. https://doi.org/10.1016/j.ijcard.2013.06.051.
Abegaz B, Davern PJ, Jackson KL, Nguyen-Huu T-P, Bassi JK, Connelly A, et al. Cardiovascular role of angiotensin type1A receptors in the nucleus of the solitary tract of mice. Cardiovasc Res. 2013;100(2):181–91. https://doi.org/10.1093/cvr/cvt183.
Colombari E, Colombari DS. NTS AT1a receptor on long-term arterial pressure regulation: putative mechanism. Cardiovasc Res. 2013;100(2):173–4. https://doi.org/10.1093/cvr/cvt217.
de Oliveira-Sales EB, Nishi EE, Boim MA, Dolnikoff MS, Bergamaschi CT, Campos RR. Upregulation of AT1R and iNOS in the rostral ventrolateral medulla (RVLM) is essential for the sympathetic hyperactivity and hypertension in the 2K-1C Wistar rat model. Am J Hypertens. 2010;23(7):708–15. https://doi.org/10.1038/ajh.2010.64.
Song K, Kurobe Y, Kanehara H, Okunishi H, Wada T, Inada Y, et al. Quantitative localization of angiotensin II receptor subtypes in spontaneously hypertensive rats. Blood Press Suppl. 1994;5:21–6.
Park CG, Leenen F. Effects of centrally administered losartan on deoxycorticosterone-salt hypertension rats. J Korean Med Sci. 2001;16(5):553–7. https://doi.org/10.3346/jkms.2001.16.5.553.
Tsutsumi K, Saavedra JM. Characterization and development of angiotensin II receptor subtypes (AT1 and AT2) in rat brain. Am J Phys Regul Integr Comp Phys. 1991;261(1):R209–R16. https://doi.org/10.1152/ajpregu.1991.261.1.R209.
Grady EF, Sechi LA, Griffin CA, Schambelan M, Kalinyak JE. Expression of AT2 receptors in the developing rat fetus. J Clin Invest. 1991;88(3):921–33. https://doi.org/10.1172/JCI115395.
Wu C-Y, Zha H, Xia Q-Q, Yuan Y, Liang X-Y, Li J-H, et al. Expression of angiotensin II and its receptors in activated microglia in experimentally induced cerebral ischemia in the adult rats. Mol Cell Biochem. 2013;382(1–2):47–58. https://doi.org/10.1007/s11010-013-1717-4.
Stern JE, Son S, Biancardi VC, Zheng H, Sharma N, Patel KP. Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension. 2016;68(6):1483–93. https://doi.org/10.1161/HYPERTENSIONAHA.116.07747.
Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui T-X, et al. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Phys Heart Circ Phys. 2003;284(1):H116–H21. https://doi.org/10.1152/ajpheart.00515.2002.
Gao L, Wang W-Z, Wang W, Zucker IH. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52(4):708–14. https://doi.org/10.1161/HYPERTENSIONAHA.108.116228.
Blanch GT, Freiria-Oliveira AH, Speretta GFF, Carrera EJ, Li H, Speth RC, et al. Increased expression of angiotensin II type 2 receptors in the solitary–vagal complex blunts renovascular hypertension. Hypertension. 2014;64(4):777–83. https://doi.org/10.1161/HYPERTENSIONAHA.114.03188.
Ruchaya PJ, Speretta GF, Blanch GT, Li H, Sumners C, Menani JV, et al. Overexpression of AT2R in the solitary-vagal complex improves baroreflex in the spontaneously hypertensive rat. Neuropeptides. 2016;60:29–36. https://doi.org/10.1016/j.npep.2016.06.006.
Wei S-G, Yu Y, Zhang Z-H, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65(5):1126–33. https://doi.org/10.1161/HYPERTENSIONAHA.114.05112This paper demonstrated pro-inflammatory actions mediated by AT1R in the cardiovascular control centers of the brain.
Yu Y, Wei S-G, Weiss RM, Felder RB. TNF-α receptor 1 knockdown in subfornical organ ameliorates sympathetic excitation and cardiac hemodynamics in heart failure rats. Am J Phys Heart Circ Phys. 2017;313(4):H744–H56. https://doi.org/10.1152/ajpheart.00280.2017.
Sriramula S, Cardinale JP, Francis J. Inhibition of TNF in the brain reverses alterations in RAS components and attenuates angiotensin II-induced hypertension. PLoS One. 2013;8(5):e63847. https://doi.org/10.1371/journal.pone.0063847.
Lu P, Jiang S-j, Pan H, Xu A-l, Wang G-h, Ma C-l, et al. Short hairpin RNA interference targeting interleukin 1 receptor type I in the paraventricular nucleus attenuates hypertension in rats. Pflügers Arch. 2017;470(2):439–48. https://doi.org/10.1007/s00424-017-2081-0.
Colombo E, Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37(9):608–20. https://doi.org/10.1016/j.it.2016.06.006.
Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV. Potential role of MCP-1 in endothelial cell tight junction ‘opening’: signaling via Rho and Rho kinase. J Cell Sci. 2003;116(22):4615–28. https://doi.org/10.1242/jcs.00755.
Stamatovic SM, Keep RF, Wang MM, Jankovic I, Andjelkovic AV. Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells. J Biol Chem. 2009;284(28):19053–66. https://doi.org/10.1074/jbc.M109.000521.
Ishibashi M, Hiasa K-i, Zhao Q, Inoue S, Ohtani K, Kitamoto S, et al. Critical role of monocyte chemoattractant protein-1 receptor CCR2 on monocytes in hypertension-induced vascular inflammation and remodeling. Circ Res. 2004;94(9):1203–10. https://doi.org/10.1161/01.RES.0000126924.23467.A3.
Setiadi A, Korim WS, Elsaafien K, Yao ST. The role of the blood–brain barrier in hypertension. Exp Physiol. 2018;103(3):337–42. https://doi.org/10.1113/EP086434.
Weidanz J, Jacobson LM, Muehrer RJ, Djamali A, Hullett DA, Sprague J, et al. AT1R blockade reduces IFN-γ production in lymphocytes in vivo and in vitro. Kidney Int. 2005;67(6):2134–42. https://doi.org/10.1111/j.1523-1755.2005.00318.x.
Xie Q-y, Sun M, Yang T-l, Sun Z-L. Losartan reduces monocyte chemoattractant protein-1 expression in aortic tissues of 2K1C hypertensive rats. Int J Cardiol. 2006;110(1):60–6. https://doi.org/10.1016/j.ijcard.2005.07.046.
Ye S, Zhong H, Duong VN, Campese VM. Losartan reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertension. 2002;39(6):1101–6. https://doi.org/10.1161/01.hyp.0000018590.26853.c7.
Yu X-J, Suo Y-P, Qi J, Yang Q, Li H-H, Zhang D-M, et al. Interaction between AT1 receptor and NF-κB in hypothalamic paraventricular nucleus contributes to oxidative stress and sympathoexcitation by modulating neurotransmitters in heart failure. Cardiovasc Toxicol. 2013;13(4):381–90. https://doi.org/10.1007/s12012-013-9219-x.
de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, et al. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33(11):4825–33. https://doi.org/10.1523/JNEUROSCI.3806-12.2013This paper demonstrated pro-inflammatory actions mediated by AT1R in the cardiovascular control centers of the brain.
Wang L, Hiller H, Smith JA, de Kloet AD, Krause EG. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus control cardiovascular reactivity and anxiety-like behavior in male mice. Physiol Genomics. 2016;48(9):667–76. https://doi.org/10.1152/physiolgenomics.00029.2016This paper demonstrated pro-inflammatory actions mediated by AT1R in the cardiovascular control centers of the brain.
Miguel-Carrasco JL, Zambrano S, Blanca AJ, Mate A, Vázquez CM. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J Inflamm. 2010;7(1):21. https://doi.org/10.1186/1476-9255-7-21.
Chen S, Ge Y, Si J, Rifai A, Dworkin LD, Gong R. Candesartan suppresses chronic renal inflammation by a novel antioxidant action independent of AT1R blockade. Kidney Int. 2008;74(9):1128–38. https://doi.org/10.1038/ki.2008.380.
Jun JY, Zubcevic J, Qi Y, Afzal A, Carvajal JM, Thinschmidt JS, et al. Brain-mediated dysregulation of the bone marrow activity in angiotensin II–induced hypertension. Hypertension. 2012;60(5):1316–23. https://doi.org/10.1161/HYPERTENSIONAHA.112.199547.
Valero-Esquitino V, Lucht K, Namsolleck P, Monnet-Tschudi F, Stubbe T, Lucht F, et al. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin Sci. 2015;128(2):95–109. https://doi.org/10.1042/CS20130601.
Bhat SA, Sood A, Shukla R, Hanif K. AT2R activation prevents microglia pro-inflammatory activation in a NOX-dependent manner: inhibition of PKC activation and p47 phox phosphorylation by PP2A. Mol Neurobiol. 2019;56(4):3005–23. https://doi.org/10.1007/s12035-018-1272-9.
Nomoto T, Okada T, Shimazaki K, Yoshioka T, Nonaka-Sarukawa M, Ito T, et al. Systemic delivery of IL-10 by an AAV vector prevents vascular remodeling and end-organ damage in stroke-prone spontaneously hypertensive rat. Gene Ther. 2009;16(3):383–91. https://doi.org/10.1038/gt.2008.151.
Sawada M, Suzumura A, Hosoya H, Marunouchi T, Nagatsu T. Interleukin-10 inhibits both production of cytokines and expression of cytokine receptors in microglia. J Neurochem. 1999;72(4):1466–71. https://doi.org/10.1046/j.1471-4159.1999.721466.x.
Yu Y, Zhang Z-H, Wei S-G, Chu Y, Weiss RM, Heistad DD, et al. Central gene transfer of interleukin-10 reduces hypothalamic inflammation and evidence of heart failure in rats after myocardial infarction. Circ Res. 2007;101(3):304–12. https://doi.org/10.1161/CIRCRESAHA.107.148940.
Segiet A, Smykiewicz P, Kwiatkowski P, Żera T. Tumour necrosis factor and interleukin 10 in blood pressure regulation in spontaneously hypertensive and normotensive rats. Cytokine. 2019;113:185–94. https://doi.org/10.1016/j.cyto.2018.07.003.
Jiang N, Shi P, Desland F, Kitchen-Pareja MC, Sumners C. Interleukin-10 inhibits angiotensin II-induced decrease in neuronal potassium current. Am J Physiol Cell Physiol. 2013;304(8):C801–C7. https://doi.org/10.1152/ajpcell.00398.2012.
Hernanz R, Martínez-Revelles S, Palacios R, Martin A, Cachofeiro V, Aguado A, et al. Toll-like receptor 4 contributes to vascular remodelling and endothelial dysfunction in angiotensin II-induced hypertension. Br J Pharmacol. 2015;172(12):3159–76. https://doi.org/10.1111/bph.13117.
Ji Y, Liu J, Wang Z, Liu N. Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem. 2009;23(4–6):265–76. https://doi.org/10.1159/000218173.
Beutler B. TLR4 as the mammalian endotoxin sensor. Toll-like receptor family members and their ligands. Springer; 2002. p. 109–20.
Acknowledgements
This was supported by the following National Institutes of Health Grants: R01HL136595(EGK and CS), R35HL150750 (EGK), R01HL139868 (EGK), and R01HL145028 (AdK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Secondary Hypertension: Nervous System Mechanisms
Rights and permissions
About this article
Cite this article
Elsaafien, K., de Kloet, A.D., Krause, E.G. et al. Brain Angiotensin Type-1 and Type-2 Receptors in Physiological and Hypertensive Conditions: Focus on Neuroinflammation. Curr Hypertens Rep 22, 48 (2020). https://doi.org/10.1007/s11906-020-01062-0
Published:
DOI: https://doi.org/10.1007/s11906-020-01062-0