Abstract
Purpose of Review
Reducing the risk of HIV-associated neurocognitive disorders (HAND) is an elusive treatment goal for people living with HIV. Combination antiretroviral therapy (cART) has reduced the prevalence of HIV-associated dementia, but milder, disabling HAND is an unmet challenge. As newer cART regimens that more consistently suppress central nervous system (CNS) HIV replication are developed, the testing of adjunctive neuroprotective therapies must accelerate.
Recent Findings
Successes in modifying cART regimens for CNS efficacy (penetrance, chemokine receptor targeting) and delivery (nanoformulations) in pilot studies suggest that improving cART neuroprotection and reducing HAND risk is achievable. Additionally, drugs currently used in neuroinflammatory, neuropsychiatric, and metabolic disorders show promise as adjuncts to cART, likely by broadly targeting neuroinflammation, oxidative stress, aerobic metabolism, and/or neurotransmitter metabolism. Adjunctive cognitive brain therapy and aerobic exercise may provide additional efficacy.
Summary
Adjunctive neuroprotective therapies, including available FDA-approved drugs, cognitive therapy, and aerobic exercise combined with improved cART offer plausible strategies for optimizing the prevention and treatment of HAND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The persistence of neurocognitive impairment (NCI) associated with HIV infection, known by the Frascati criteria as HIV-associated cognitive disorders (HAND), or more generally as HIV-NCI, in the era of suppressive combination antiretroviral therapy (cART) is a challenge for people with HIV (PWH) [1, 2]. Although suppression of HIV replication reduces the risk for severe HIV-NCI, the effects of comorbid conditions, viral “blipping” or escape, chronic neuroinflammation and oxidative stress, and persistent effects of early injury in acute HIV infection (before cART achieves full suppression) probably contribute to long-term HIV-NCI risk [3, 4•]. Complete neuroprotection and reduction of HIV-NCI risk has not yet been achieved [5•]. Because HIV replication is the initiating factor for HIV-NCI, neuroprotection approaches must include complete suppression of HIV replication and HIV gene expression with modified cART regimens, and also modulation of cell/virus trafficking and spread, disordered metabolism, and inappropriate activation of oxidative stress and inflammation pathways that impact neuronal cell function.
Among comprehensive studies of the prevalence of HIV-NCI is the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) cohort study [6]. Cognitive impairment (all causes) was found in 814 (52%) of 1555 (PWH) attending treatment centers in the USA [6]. Since the introduction of cART, the prevalence of severe HIV-NCI (HIV-associated dementia) has been reduced from approximately 20% in the pre-ART era to approximately 2–5% [6]. This reduction of HIV-NCI severity by cART represents the only consistent treatment success to date (reviewed in [7]). Unfortunately, less severe HIV-NCI is nonetheless associated with disabling functional impairment in activities of daily living in 10–12% of PWH [2, 6]. This morbidity burden will likely grow as the population of PWH ages, emphasizing a need for additional treatment strategies to improve brain protection in PWH.
This review discusses neuroprotective strategies to improve neurocognitive outcomes in PWH, for which the foundation is suppressive cART (Table 1, Fig. 1). More effective neuroprotection strategies will require the continued evolution of cART regimens and the development of adjunctive therapies. Many preliminary successes in reducing HIV-NCI have been seen in small pilot clinical trials, which have often later been recognized as treatment failures when examined in larger well-controlled validation studies. Nonetheless, as knowledge of the functional, biochemical, and structural integrity of the CNS in PWH continues to grow, and as new neuroprotective/neuromodulating medications and drug delivery systems are developed for other CNS disorders, future success seems likely. This review will target selected areas of cART strategies, therapies for neuroinflammation and oxidative stress, neurodegenerative disease therapies, neuropsychiatric medications, trophic factors, and exercise therapy, with the recognition that some areas of potential interest are outside of the selected scope of this review.
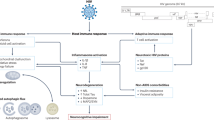
Proposed sites of neuroprotective drug effects in HIV neuropathogenesis. Multiple steps in HIV neuropathogenesis are potentially targetable for neuroprotection. Acute HIV infection results in (1) structural damage to the gut mucosal barrier and translocation of immune-activating microbial products into the systemic circulation, (2) transendothelial migration of HIV-infected immune cells into the CNS, and (3) HIV replication within perivascular/parenchymal macrophages and microglia and trafficking T lymphocytes. Amplification of pro-inflammatory and oxidative processes in infected and non-infected activated glial cells is associated with release of toxic metabolic products, including reactive oxygen species, pro-inflammatory cytokines, HIV proteins, and excitotoxic neurotransmitters. Abbreviations: INSTIs—integrase strand transfer inhibitors; DMF—dimethyl fumarate; SSRIs—selective serotonin re-uptake inhibitors
Improving cART Regimens
Improving ART effectiveness in suppressing HIV replication in the CNS by either increasing penetration into the CNS or intensifying ART through addition of other classes of drugs is a strategy for reducing mild-moderate HIV-NCI risk. Improved penetration or retention of bioavailable ART drugs in the CNS might decrease CNS HIV replication and reduce concomitant release of neurotoxins from infected and activated macrophages/microglia and immune-activated astrocytes [8••, 9]. Furthermore, choosing ART regimens based upon their efficacy in reducing HIV replication in monocyte and macrophage lineages may be more efficacious in targeting HIV-NCI pathogenesis [10].
Maintaining Viral Control: the Problem of Cerebrospinal Fluid Viral “blipping”
Despite the general effectiveness of cART in suppressing HIV replication, a surprisingly high rate (10–20%) of HIV “blipping” (defined as spontaneous expression of HIV RNA copies in CSF in the setting of undetectable viral RNA in plasma) is observed [11]. This suggests that cART does not maintain consistent long-term viral suppression within the CNS reservoir, and that “blipping” might be a risk factor for persistent HIV-NCI risk in virally suppressed PWH [12]; thus, development of new, more consistently suppressive cART regimens may be required for more effective neuroprotection [13]. The suppression of HIV blipping should be considered as a goal for improving cART efficacy in reducing risk for HIV-NCI, through enhancing CNS ART delivery.
Enhancing ART CNS Penetrance
Classification of ART drugs by the CNS penetrance efficacy (CPE) ranking has been developed to rank individual drugs according to their ability to penetrate into the CNS [14, 15]. Although application of favorable CPE-ranked ART regimens has shown promise in reducing CSF expression of markers of neuroinflammation, CSF HIV load, and incidence or progression of HIV-NCI in patients in some studies, enhanced efficacy of cART regimens based upon the CPE has not been consistently observed [16, 17, 18••]. However, cumulative evidence does associate some high-CPE regimens with higher CNS HIV suppression and a possible benefit for HIV-NCI, thus supporting additional, larger-scale investigations [18••].
The inception of nanoparticulate-ART (nanoART), ART nanosuspensions, and associated monocyte/macrophage targeted ART strategies has also produced promising results, at least in humanized HIV-infected mice, which suggests that enhancing CNS ART delivery can have neuroprotective effects [19–21]. These and other re-formulated ART preparations that produce longer-lasting effective drug release may soon be ready for new human clinical trials for HIV-NCI neuroprotection [22, 23].
ART Intensification with Chemokine Receptor Blockade
The chemokine receptor CCR5, which is expressed at high levels on activated T lymphocytes and macrophages, serves as an HIV-1 co-receptor and regulator of chemotaxis. Maraviroc, a CCR5 chemokine receptor antagonist and entry inhibitor, has been investigated as an ART intensification drug for reduction of HIV-NCI risk, with promising early results [24, 25]. Although a large-scale maraviroc intensification study for HIV-NCI risk reduction has not been developed, there is enthusiasm for targeting CCR5 for treatment in neuroinflammatory disorders in general, and further investigations of maraviroc as a neuroprotectant in HIV-NCI could be justified [26••].
In contrast, cenicriviroc is a blocker of CCR5 and CCR2 chemokine receptors, thereby not only blocking HIV entry into cells, but also inflammation and monocyte migration [27]. CCL2 (the cognate ligand for CCR2) is a chemotactic chemokine that recruits CD14 + CD16 + monocytes across the blood–brain barrier. CCL2 is elevated in the CNS of HIV-infected individuals with HIV-NCI, even those treated with ART (reviewed in [28]). A pilot 24-week trial of cenicriviroc in 17 cART-suppressed PWH with mild to moderate HIV-NCI showed some modest beneficial effect [29•]. Thus, the potential neuroprotective effects of combination blockade of chemokine receptors (CCR5, CCR2) involved in HIV entry and those involved with chemotaxis should be further considered in larger prospective studies.
Integrase Strand Transfer Inhibitors (INSTI)
Integrase strand transfer inhibitors (INSTI) can effectively block the final ligation (integration) of the HIV provirus into cellular DNA, thereby limiting productive HIV replication. INSTI are widely used, effective, and generally well-tolerated, although weight gain may be associated with their use [30••, 31•]. In recent studies, inclusion of INSTI, including dolutegravir (DTG), in cART regimens in early HIV treatment did not result in improved neurocognitive performance or neuroimaging assessments of brain integrity [32•, 33•, 34••]. Additionally, a recent 96-week, randomized, placebo-controlled intensification study (ACTG 5234; 25 national and international sites; ClinicalTrials.gov Identifier: NCT02519777) examined the addition of DTG with or without maraviroc to suppressive ART. Unlike previous studies, this study included a DTG arm alone, and it was randomized, double-blinded, and placebo-controlled. The study showed no significant difference from placebo effects on neurocognitive performance in 191 PWH (https://www.natap.org/2022/CROI/croi_187.htm). Notably, there was no worsening of symptoms of depression on those individuals receiving DTG alone, which is of considerable interest in view of reports of adverse neuropsychiatric effects of DTG [34••, 35]. Thus, although no new ART-improvement INSTI strategies that consistently increase ART efficacy in reducing HIV-NCI risk have been developed, there is need for further study of INSTI. Overall, the strategy of increasing cART CNS effectiveness and reducing CNS ART toxicity risks are worthy goals to develop as newer ART drugs of all classes are brought forth.
ART Toxicity
The possibility of long-term ART neurotoxicity is inescapable, and reducing such risk without sacrificing ART suppressive efficacy is challenging [35]. Long-term treatment with ART is associated with a range of systemic toxicities including hepatic steatosis, peripheral neuropathy, cardiomyopathy, pancreatitis, ototoxicity, retinopathy, and lipodystrophy. Substantial toxicity of various nucleoside reverse transcriptase inhibitors (NRTIs) in different tissue types has been observed, and ART toxicity within the CNS compartment is a major concern. In vitro studies demonstrate that numerous ART drugs are directly neurotoxic at concentrations detected in the CSF of PLWH on cART, and certain cART regimens, including those with INSTI, have been associated with poorer neurocognitive outcomes [35–37]. Concerns over the potential risk for dolutegravir (DTG) use during pregnancy as an inducer of neural tube defects have proved to be unfounded, however [38••, 39••]. Continual surveillance of cognitive functioning in PLWH as cART regimens evolve is essential for defining and ultimately mitigating toxicity risks, which are clearly superseded by the neuroprotective effects of cART.
Inflammation and Oxidative Stress in HIV-NCI
Neuroinflammation
In ART-naïve subjects, HIV infection of the brain produces robust expression of pro-inflammatory cytokines and chemokines, reactive oxygen species, glutamate and other non-proteinaceous neurotoxins, and perhaps some HIV proteins; each of these has been linked to disrupted neuronal function and architecture [40, 41]. Inflammatory mediators modulate the permissibility of the blood–brain barrier and the entry of infected lymphocytes and monocytes into the CNS. Additionally, inflammatory processes in the periphery, such as gut permeability and bacterial translocation, have been linked to HIV-NCI [42, 43]. Therefore, reducing inflammation in the periphery as well as within the CNS could be expected to reduce HIV-NCI risk in PLWH. However, consistency of expression of these inflammation factors and their associated linkage to neuronal injury and dysfunction in individuals on suppressive cART is not as clear, and this thesis has been challenged [44]. Regardless of whether persistent neuroinflammation and/or intermittent neuroinflammation associated with HIV CSF blipping in cART-suppressed patients drive HIV-NCI pathogenesis, each should be further investigated both in PWH and in non-human primate models of suppressed SIV infection to meet this challenge [45].
Oxidative Stress
Oxidative stress is considered to be an imbalance between oxidants and antioxidant defenses, and it manifests as oxidative damage to proteins, nucleic acids, and other biomolecules, with resulting cellular injury. Oxidative stress and inflammation are closely related processes that often co-exist in chronic diseases, and neuroprotection strategies should target both processes concurrently [46]. HIV infection induces pathological oxidative stress, as evidenced by diminished levels of reduced glutathione in plasma, lymphocytes, and PBMC and elevated levels of biomarkers of lipid peroxidation products such as malondialdehyde and hydroperoxide; several of these and other markers of oxidative stress correlate with disease progression and mortality [47–51]. Oxidative stress can in turn drive inflammation through enhancing NF-κB-driven HIV replication and release of proinflammatory cytokines [52–55].
Among the damaging molecules involved in oxidative injury are free radicals (superoxide anion, nitric oxide, hydroxyl radical), reactive oxygen species (including free radicals and non-radicals such as H2O2 and peroxynitrite), and reactive nitrogen species [46, 56]. Suppression of HIV replication with cART significantly reduces immune activation and oxidative stress; however,this suppression is incomplete [57–59]. This suggests that the pathways driving production of neurotoxins remain active and that neuronal damage can accumulate even during virologic suppression. Despite some previous therapeutic failures in treating HIV-NCI with free radical scavenging drugs, newer drugs with broader targeting of inflammation and oxidative stress pathways may offer new options worth pursuing [8••].
Contribution of Systemic Inflammation: the Gut
HIV infection is associated with translocation of microbial products across a damaged gastrointestinal tract and dysbiosis of bacterial populations, although a recent study has questioned the association between HIV infection and altered gut mucosal integrity in PLWH [60–63]. Decreased microbial diversity and alterations of abundance of different genera, including increased abundance of the genus Prevotella and decreased prevalence of the genus Bacteroides, are characteristic of HIV infection [62]. Generally, changes towards decreased abundance of commensal, protective bacteria and increased abundance of pro-inflammatory bacteria are observed, with many variations in specific genera. Increased systemic inflammation has been linked to the translocated microbial product LPS, which is elevated in the plasma, but not the CSF, of PWH [64•]. Plasma LPS associates with monocyte activation, indicated by elevated blood levels of soluble CD14 (sCD14) and sCD163, and elevated expression of monocyte-associated CD14 and CD16 [65]. Elevated blood levels of CD16 + monocytes, sCD14, sCD163 and total CD14 + monocyte HIV DNA content associated with microbial translocation have been correlated with an increased risk for HIV-NCI. Therefore, the gut microbiome and LPS are considered targets for reducing pathogenic effects of microbial translocation [66].
Although targeting of LPS (e.g., through sequestration with the drug sevelamer) has not demonstrated benefits for cardiovascular dysfunction associated with inflammation in PWH, targeting LPS has not yet been explored in HIV-NCI risk reduction. Nonetheless, it should be considered [62]. Also, therapies targeted towards directly reducing microbial translocation (e.g., preserving gut mucosal integrity) in PLWH may have a role in neuroprotection, although no such specific therapies currently exist [67]. Focusing on correcting the gut microbiome dysbiosis as a means of promoting gut integrity and additionally reducing systemic inflammation is a reasonable strategy for attempting to reduce HIV-NCI risk; indeed, preliminary evidence suggests that probiotic dietary supplementation may reduce neuroinflammation and promote neurocognitive recovery in PLWH [67–69].
Targeting Oxidative Stress and Associated Inflammation
To date, results of clinical trials with agents that, to varying degrees, target inflammation with or without oxidative stress [OPC-14117 (free radical scavenger), CPI-1189 (TNFα blocker), selegiline (MAO-B inhibitor), minocycline (antibiotic, antioxidant), paroxetine (SSRI), lexipafant (platelet activating factor receptor blocker), maraviroc (CCR5 blocker)] as a strategy for reducing HIV-NCI risk have failed to clearly confirm benefits [5•, 8••, 70••]. Nonetheless, consistent evidence for chronic inflammation, which is linked to oxidative stress in multiple body compartments in PLWH, supports a rationale for continuing efforts to test more robust therapeutics for reducing chronic inflammation (in combination with oxidative stress) to lower the risk for HIV-NCI [71]. Previous treatment failures with agents that are either relatively highly selective in their targets, or relatively weakly effective, suggests that broader targeting of inflammatory and oxidative pathways with broader-spectrum agents should be considered, particularly those already effectively used to treat other neuroinflammatory diseases.
Broadening the Target for Better Efficacy: the Host Antioxidant (ARE-driven) Response
The host protective antioxidant response is mediated in part through activation of the transcription factor NF-E2-related factor 2 (Nrf2), which drives the antioxidant response element (ARE), a cis-acting regulatory sequence in the promoter region of numerous antioxidant and anti-inflammatory genes [72]. Among these Nrf2 targets are several antioxidant enzymes, including heme oxygenase-1 (HO-1), an inducible, detoxifying enzyme that is critical for limiting oxidative stress, inflammation, and cellular injury within the CNS and other tissues. Transcriptional regulation of HO-1 depends partly upon HMOX1 promoter region genetic variations, including a (GT)n dinucleotide repeat, which associate higher basal HMOX1 transcriptional activity and inducibility, and better outcomes in inflammatory and oxidative stress–associated diseases [72–74]. We identified HO-1 as a key marker and potential regulator of HIV-NCI, and further showed that the presence of one or more short HO-1(GT)n repeat alleles, which are known to have higher HO-1 transcriptional activity, associate with a decreased risk for HIV neuroinflammation and HIV NCI [75–78, 79•]. Furthermore, our studies suggest that African-Americans may be more vulnerable to HIV NCI than others because of differences in their HO-1 (GT)n allele genotype prevalence [79•]. Reduced risk for HIV-NCI and its associated neuroinflammation in PWH with common HMOX1 genetic variations argues strongly for a role for HO-1, among other ARE-driven antioxidant enzymes, as appealing therapeutic targets for HIV-NCI risk reduction.
Nrf2 Activators: Targeting the ARE Antioxidant Response
Therapeutic targeting of ARE-driven gene expression to enhance antioxidant responses and associated inflammation cascades has proved successful in the treatment of multiple sclerosis, and it is proposed as a strategy for prevention of HIV-NCI [71, 75, 80]. The Nrf2 transcriptional activator, dimethyl fumarate (DMF), is an FDA-approved treatment for multiple sclerosis, with excellent CNS penetration and efficacy in reducing neuroinflammation in demyelinating plaques, clinical relapse rates, and accumulation of new demyelinating lesions [81]. DMF, and its primary in vivo metabolite monomethyl fumarate (MMF), effectively induce HO-1 expression, inhibit NFκB nuclear translocation, and attenuate macrophage-mediated neurotoxicity in in vitro models of HIV neurotoxicity [82, 83]. Furthermore, DMF suppresses the Warburg effect (proinflammatory induction of aerobic glycolysis) in the mammalian brain, which may underlie its neuroprotective effect in multiple sclerosis patients [84••]. Daily oral delivery (> 100 days) of DMF to SIV-infected rhesus macaques resulted in concordantly increased expression of ARE-driven antioxidant enzymes (including HO-1) and reduced oxidative DNA and protein damage in multiple brain regions, compared with untreated, non-infected macaques [85•]. The efficacy of DMF in multiple sclerosis patients, in combination with in vitro and in vivo studies of HIV/SIV neuroprotection makes it an attractive candidate for HIV-NCI neuroprotection studies. Additionally, several other Nrf2-activating drugs (bardoxolone, resveratrol, others) are also being examined in multiple clinical trials for various inflammatory/oxidative disease states, further supporting Nrf2-directed therapeutics as a promising target for HIV-NCI neuroprotection trials [86•].
Statins
Statins are routinely used as lipid-lowering agents that act via blocking hydroxymethlyglutaryl-CoA (HMG-CoA) enzymatic activity. They also express other pleiotropic anti-inflammatory, antioxidant, and immunomodulatory effects, which make them attractive for tissue protection in a variety of disease states [87•]. High-dose (80 mg daily) atorvastatin has been shown to reduce monocyte activation in cART-treated PLWH, but beneficial effects on HIV-NCI risk have not been observed [88, 89]. A review of randomized controlled trials and observational studies of various study designs not involving PLWH indicates that initiation of statin therapy late in life does not prevent cognitive decline or dementia over 3–5 ensuing years [90]. Although some studies have indicated a protective effect against acute brain injury, studies in multiple sclerosis patients (in which neuroinflammation and oxidative stress are major pathogenic mechanisms) have also failed to demonstrate protective effects of statins [91, 92•]. This stands in contrast to the protective effects of DMF in multiple sclerosis patients, which suggests that more robust induction of Nrf2-driven anti-inflammatory and oxidative stress pathways is necessary for neuroprotection. Thus, lacking any additional evidence for statin neuroprotective effects in humans, the likelihood of beneficial effects of statins on HIV-NCI risk appears low.
Other Therapies Applied to Neurodegenerative Diseases and Neuropsychiatric Disorders
Neurodegenerative Disease Therapies
Several aforementioned therapies used in neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease) are directed towards neuroprotection while others are directed towards treating symptoms. Memantine is approved in the treatment for Alzheimer’s disease and acts as a non-competitive NMDA glutamate receptor antagonist. A short-term clinical memantine trial in HIV-NCI patients provided neuroimaging evidence (magnetic resonance spectroscopy) of some preservation of neuronal integrity, but without significant neurocognitive improvement [93]. A longer-term follow-up failed to reveal a clinically demonstrable neurocognitive benefit, and so additional studies of memantine for HIV-NCI are probably not warranted [94].
Selegiline, a monoamine oxidase B (MAO-B) inhibitor used in the treatment of early-stage Parkinson’s disease, is proposed to act as a neuroprotectant by reducing the antioxidant burden of the cell [95]. However, clinical studies have shown neither a reduction in markers of oxidative stress nor improvement in cognitive performance with short-term (24 weeks) transdermal selegiline, thus arguing against additional selegiline efficacy trials for HIV-NCI [96, 97].
Neuropsychiatric Drug Therapies
Among drugs used in the management of neuropsychiatric disorders, sodium valproate (VPA) and lithium are approved for treatment of bipolar disorder and related mood disorders; each inhibits glycogen synthase kinase-3β and provides neuroprotection against HIV-induced toxicity in vitro and in mouse models [98–100]. Although several open-label pilot studies of lithium therapy demonstrated improved neurocognitive performance in PLWH with HIV-NCI, a more recent randomized, placebo-controlled study of lithium showed no beneficial effect on HIV-NCI [101–103]. In an observational study of VPA use in PLWH over an average of 18 months, VPA users vs. non-users demonstrated poorer neurocognitive performance [104]. Subsequently, a placebo-controlled pilot study of VPA also failed to demonstrate cognitive improvement in PLWH and with HIV-NCI[105]. It is important to note that VPA is also a histone deacetylase inhibitor (HDAC), and thus there is at least a theoretical risk of reactivating HIV expression from a state of latency, which could be a potential confounder in identifying possible neuroprotective effects in vivo. Nonetheless, available clinical trial data to date do not convincingly support further trials for either lithium or VPA for HIV-NCI reduction risk.
Selective serotonin reuptake inhibitors (SSRIs) have gained increasing attention for their beneficial effects on depression and their relatively limited side effects in PLWH [106••, 107]. Additionally, some benefit of SSRIs in reducing immune activation may be seen. Within the Veterans Aging Cohort Study, investigators showed significant, albeit modest, reduction in plasma CD14 and IL-6 in SSRI users, among 1546 HIV-positive veterans [108•]. However, very limited research has been conducted to prospectively evaluate SSRIs as adjunctive therapies for HIV-NCI [109–111]. A recent double-blind, placebo-controlled study of 45 PWH sub-divided into patient groups receiving paroxetine and/or fluconazole over 24 weeks showed significant improvement in four neuropsychological tests (including the NPZ8 summary measure), with worse performance in two neuropsychological tests, in those receiving paroxetine [111]. Considering the results of this first-of-its kind, controlled study of SSRI and HIV-NCI outcomes in PLWH, additional SSRI studies should be considered. At least one such study has been proposed [112].
Other Therapies Targeting Cell Trafficking and Trophic Factors
Cell Trafficking
Limiting trafficking of HIV-infected cells into various tissue compartments, including the CNS, may be considered as a possible neuroprotective strategy, although this line of research is primarily directed towards limiting the size of the HIV reservoir in lymphoid tissues. Fingolimod (FTY720), an FDA-approved treatment for multiple sclerosis, is a lysophospholipid sphingosine-1 phosphate receptor modulator that limits egress of CD4 + and CD8 + T lymphocytes from lymph nodes [113•]. A pilot study in SIV-infected rhesus macaques showed reduction in SIV DNA-containing lymphocytes in lymph nodes in fingolimod-treated animals, presumably reflecting the action of retained nodal cytotoxic T lymphocytes [114•]. No studies of CNS effects of fingolimod in SIV-infected macaques have been published. Although fingolimod is currently used for treating multiple sclerosis, recent concerns about the possibility of reduced response to SARS CoV-2 vaccination may temper enthusiasm for expanding neuroprotection studies to HIV-NCI [115].
Natalizumab, an FDA-approved treatment for multiple sclerosis, is an anti-α4 integrin-targeting monoclonal antibody that inhibits monocyte and T-lymphocyte trafficking to the brain and other tissues [116, 117]. In the rhesus macaque model of multiple sclerosis (experimental autoimmune encephalomyelitis), natalizumab blocked inflammation, demyelination, and ingress of monocytes and T lymphocytes into the CNS [116]. In a pilot study of SIV infection of rhesus macaques, natalizumab treatment of prior to SIV inoculation prevented CNS SIV infection, while treatment post-SIV infection significantly reduced progressive SIV-associated neuronal injury [118]. These data suggest that natalizumab could have some neuroprotective effect against ongoing neuronal injury secondary to continuing immune cell trafficking into the CNS in PLWH. However, the relatively high-risk of reactivation of JC virus and associated genesis of progressive multifocal leukoencephalopathy with prolonged treatment with natalizumab, compared to other treatments in multiple sclerosis patients, will also likely reduce enthusiasm for neuroprotection studies in HIV-NCI.
Trophic Agents Targeting Metabolism: Intranasal Insulin
To date, published studies of clinical investigations of trophic factors for the treatment of HIV-NCI are limited to the use of intranasal insulin. In patients without HIV, administration of insulin intranasally has been associated with improved cognitive performance in patients with diabetes mellitus, while a randomized clinical trial in patients with Alzheimer’s disease showed no benefit [119•, 120•]. A preliminary report of a pilot study (ClinicalTrials.gov Identifier: NCT03081117) of intranasal insulin administration for the treatment of HIV-NCI in 21 PWH indicated improvement in neuropsychological tests of memory and attention (https://www.natap.org/2021/CROI/croi_91.htm). A full report of clinical and biomarker outcomes in this pilot study is anticipated (Norman Haughey, personal communication).
Alternative Adjunctive Therapies: Cognitive Therapy and Exercise
Non-pharmacologic interventions such as cognitive therapy and exercise may have a role in improving HIV-NCI through direct or indirect effects [121, 122•, 123•]. Cognitive training in PWH has demonstrated beneficial effects in specific cognitive domains that are targeted by the training, and an interventional clinical trial with individualized targeting of specific domains of deficit in 109 PWH has been completed [123•, 124] (ClinicalTrials.gov Identifier: NCT03122288). In general, the neuroprotective effects of aerobic exercise on structural brain integrity have been well-documented, but whether cognitive therapy also has a protective effect on brain structural integrity is not known [125, 126]. In PWH, physical exercise indeed associates with lower risk for HIV-NCI [122•, 127]. A longitudinal study of 291 PWH demonstrated that individuals with consistent physical activity (physical activity and ≥ 50% of study visits) maintained better neurocognitive functioning in domains of verbal fluency, working memory, speed of information processing executive function, and motor function over a mean period of 35 months, even when adjusting for confounding factors [122•]. Initiating cognitive training with regular aerobic exercise is a low-cost, low risk strategy that should be combined with adjunctive pharmacologic neuroprotection strategies in PWH for reducing risk for HIV-NCI.
Conclusions
The persistent risk of HIV-NCI in PLWH despite suppressive cART requires improvement of cART CNS effectiveness and the implementation of adjunctive therapies for risk reduction based upon preservation of brain integrity. Lessons from past clinical trials suggest that early intervention with agents that more broadly target relevant pathogenic pathways effects should be prioritized, and among attractive targets are pathways of inflammation, oxidative stress, neurotransmitter metabolism, and metabolism-modulating trophic factors. Minimizing effects of impaired gut mucosal integrity and altered gut microflora with microbiome modifications also holds future promise. For drug therapies, it is likely that concurrent initiation of an adjunctive therapeutic drug in combination with cART in acute HIV infection may have the most profound effect, and even short-duration treatment may have long-term benefits. Agents that robustly activate endogenous antioxidant response genes while suppressing associated inflammatory responses should be pursued, and one example is dimethyl fumarate, widely used in the treatment of multiple sclerosis. Other currently approved neuromodulating drugs that limit immune cell trafficking into the CNS in multiple sclerosis patients are probably too risky for use in PWH. Re-purposing of other available drugs, including SSRIs such as paroxetine, which also directly or indirectly suppress inflammatory and oxidative stress processes, deserves immediate attention. Development of new pharmacological agents targeting these and other newly identified pathological pathways should also be pursued as a longer-term strategy. Each approach should be combined with aerobic exercising and cognitive therapy, which can be promoted by individualized lifestyle training. In total, these approaches will likely also mitigate effects of comorbidities that contribute to HIV-NCI and enhance the quality of life for PWH.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99.
Wang Y, Liu M, Lu Q, et al. Global prevalence and burden of HIV-associated neurocognitive disorder: a meta-analysis. Neurology. 2020;95:e2610–21.
Heaton RK, Franklin DR Jr, Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis. 2015;60:473–80.
• Chan P, Kerr SJ, Kroon E, et al. Cognitive trajectories after treatment in acute HIV infection. AIDS. 2021;35(6):883–8. https://doi.org/10.1097/QAD.0000000000002831. Immediate suppressive ART in AHI improves and sustains cognitive function in multiple domains for up to 6 years, with the largest improvements in patients with the poorest baseline cognitive functioning. Provides support for earliest possible intervention with cART, and suggests possible window of opportunity for early adjunctive therapies.
• Bougea A, Spantideas N, Galanis P, et al. Optimal treatment of HIV-associated neurocognitive disorders: myths and reality. A critical review. Ther Adv Infect Dis. 2019;6:2049936119838228. This is an up-to-date, comprehensive review that adds important perspective to treatment of HIV-NCI.
Heaton RK, Clifford DB, Franklin DR Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96.
Nightingale S, Winston A, Letendre S, et al. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014;13:1139–51.
•• Lin SP, Calcagno A, Letendre SL, et al. Clinical treatment options and randomized clinical trials for neurocognitive complications of HIV infection: combination antiretroviral therapy, central nervous system penetration effectiveness, and adjuvants. Curr Top Behav Neurosci. 2021;50:517–545. A very comprehensive review of cross-sectional, prospective, and randomized controlled trials of CPE-rated regimens in HIV-NCI.
Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44.
Shikuma CM, Nakamoto B, Shiramizu B, et al. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir Ther. 2012;17:1233–42.
Grennan JT, Loutfy MR, Su D, et al. Magnitude of virologic blips is associated with a higher risk for virologic rebound in HIV-infected individuals: a recurrent events analysis. J Infect Dis. 2012;205:1230–8.
Chen MF, Gill AJ, Kolson DL. Neuropathogenesis of HIV-associated neurocognitive disorders: roles for immune activation, HIV blipping and viral tropism. Curr Opin HIV AIDS. 2014;9:559–64.
Eden A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis. 2010;202:1819–25.
Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70.
Cusini A, Vernazza PL, Yerly S, et al. Higher CNS penetration-effectiveness of long-term combination antiretroviral therapy is associated with better HIV-1 viral suppression in cerebrospinal fluid. J Acquir Immune Defic Syndr. 2013;62:28–35.
Dahl V, Lee E, Peterson J, et al. Raltegravir treatment intensification does not alter cerebrospinal fluid HIV-1 infection or immunoactivation in subjects on suppressive therapy. J Infect Dis. 2011;204:1936–45.
Llibre JM, Buzon MJ, Massanella M, et al. Treatment intensification with raltegravir in subjects with sustained HIV-1 viraemia suppression: a randomized 48-week study. Antivir Ther. 2012;17:355–64.
•• Force G, Ghout I, Ropers J, et al. Improvement of HIV-associated neurocognitive disorders after antiretroviral therapy intensification: the Neuro+3 study. J Antimicrob Chemother. 2021;76:743–752. A relatively small (n=49 patients), but well-designed prospective ART intensification study of several different CPE-based ART regimens.
Dou H, Grotepas CB, McMillan JM, et al. Macrophage delivery of nanoformulated antiretroviral drug to the brain in a murine model of neuroAIDS. J Immunol. 2009;183:661–9.
Gomes MJ, Neves J, Sarmento B. Nanoparticle-based drug delivery to improve the efficacy of antiretroviral therapy in the central nervous system. Int J Nanomed. 2014;9:1757–69.
Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine (Lond). 2009;4:557–74.
Edagwa B, McMillan J, Sillman B, et al. Long-acting slow effective release antiretroviral therapy. Expert Opin Drug Deliv. 2017;14:1281–91.
McMillan J, Szlachetka A, Slack L, et al. Pharmacokinetics of a long-acting nanoformulated dolutegravir prodrug in rhesus macaques. Antimicrob Agents Chemother. 2017;62(1):e01316–17. https://doi.org/10.1128/AAC.01316-17.
Ndhlovu LC, Umaki T, Chew GM, et al. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND). J Neurovirol. 2014;20:571–82.
Gates TM, Cysique LA, Siefried KJ, et al. Maraviroc-intensified combined antiretroviral therapy improves cognition in virally suppressed HIV-associated neurocognitive disorder. AIDS. 2016;30:591–600.
•• Martin-Blondel G, Brassat D, Bauer J, et al. CCR5 blockade for neuroinflammatory diseases--beyond control of HIV. Nat Rev Neurol. 2016;12:95–105. This thought-provoking review presents a thorough insight into the multiple potential mechanisms through which CCR5 blockade could provide neuroprotection in both infectious and non-infectious neuroinflammatory diseases.
Leon-Rivera R, Veenstra M, Donoso M, et al. Central nervous system (CNS) viral seeding by mature monocytes and potential therapies to reduce CNS viral reservoirs in the cART era. mBio. 2021;12(2):e03633–20. https://doi.org/10.1128/mBio.03633-20.
Williams DW, Veenstra M, Gaskill PJ, et al. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res. 2014;12:85–96.
• D'Antoni ML, Paul RH, Mitchell BI, et al. Improved cognitive performance and reduced monocyte activation in virally suppressed chronic HIV after dual CCR2 and CCR5 antagonism. J Acquir Immune Defic Syndr. 2018;79:108–116. An open-label 24-week study demonstrating improved NP test performance and reduced monocyte activation in virally suppressed patients treated with cenicriviroc.
•• Christensen BL, Tan DH. An up-to-date evaluation of dolutegravir/abacavir/lamivudine for the treatment of HIV. Expert Opin Pharmacother. 2022;23:439–446. A truly comprehensive up-to-date summary of DTG/ABC/3TC efficacy and where this regimen stands as a first-line treatment for HIV.
• Diggins CE, Russo SC, Lo J. Metabolic consequences of antiretroviral therapy. Curr HIV/AIDS Rep. 2022;19(2):141–53. https://doi.org/10.1007/s11904-022-00600-6. A very useful review of adverse metabolic effects of current ART regimens
• O'Halloran JA, Cooley SA, Strain JF, et al. Altered neuropsychological performance and reduced brain volumetrics in people living with HIV on integrase strand transfer inhibitors. AIDS. 2019;33:1477–1483. A large (n=202 patients), provocative cross-sectional neuroimaging study suggesting reduced brain integrity in INSTI users.
• Prats A, Martinez-Zalacain I, Mothe B, et al. Effects of integrase inhibitor-based antiretroviral therapy on brain outcomes according to time since acquisition of HIV-1 infection. Sci Rep. 2021;11:11289. A prospective, controlled study of men LWH with matched seronegative controls. INSTI initiation before 3 months or later than 6 months after HIV infection associated with no cognitive differences, but with less loss of brain integrity.
•• Chan P, Goh O, Kroon E, et al. Neuropsychiatric outcomes before and after switching to dolutegravir-based therapy in an acute HIV cohort. AIDS Res Ther. 2020;17:1. A large (n = 254) AHI patients in the RV254 Thai cohort that suggests depressive symptoms associate with DTG use.
Lanman T, Letendre S, Ma Q, et al. CNS neurotoxicity of antiretrovirals. J Neuroimmune Pharmacol. 2019;16(1):130–43. https://doi.org/10.1007/s11481-019-09886-7.
Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18:388–99.
Marra CM, Zhao Y, Clifford DB, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–66.
•• Pereira GFM, Kim A, Jalil EM, et al. Dolutegravir and pregnancy outcomes in women on antiretroviral therapy in Brazil: a retrospective national cohort study. Lancet HIV. 2021;8:e33-e41. An important, large retrospective outcomes study of DTG use in pregnancy, demonstrating no DTG risk for neural tube defects.
•• Abrams E, Myer L. Lessons from dolutegravir and neural tube defects. Lancet HIV. 2021;8:e3-e4. A commentary that convincingly argues in favor of the benefits of DTG use during pregnancy.
Uzasci L, Nath A, Cotter R. Oxidative stress and the HIV-infected brain proteome. J Neuroimmune Pharmacol. 2013;8:1167–80.
McArthur JC, Johnson TP. Chronic inflammation mediates brain injury in HIV infection: relevance for cure strategies. Curr Opin Neurol. 2020;33:397–404.
Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71.
Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS ONE. 2008;3:e2516.
Price RW, Spudich SS, Peterson J, et al. Evolving character of chronic central nervous system HIV infection. Semin Neurol. 2014;34:7–13.
Stam AJ, Nijhuis M, van den Bergh WM, et al. Differential genotypic evolution of HIV-1 quasispecies in cerebrospinal fluid and plasma: a systematic review. AIDS Rev. 2013;15:152–61.
Biswas SK. Does the Interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:5698931.
Mollace V, Nottet HS, Clayette P, et al. Oxidative stress and neuroAIDS: triggers, modulators and novel antioxidants. Trends Neurosci. 2001;24:411–6.
Valcour V, Shiramizu B. HIV-associated dementia, mitochondrial dysfunction, and oxidative stress. Mitochondrion. 2004;4:119–29.
Malvy DJ, Richard MJ, Arnaud J, et al. Relationship of plasma malondialdehyde, vitamin E and antioxidant micronutrients to human immunodeficiency virus-1 seropositivity. Clin Chim Acta. 1994;224:89–94.
Suresh DR, Annam V, Pratibha K, et al. Total antioxidant capacity–a novel early bio-chemical marker of oxidative stress in HIV infected individuals. J Biomed Sci. 2009;16:61.
Wanchu A, Rana SV, Pallikkuth S, et al. Short communication: oxidative stress in HIV-infected individuals: a cross-sectional study. AIDS Res Hum Retroviruses. 2009;25:1307–11.
Israel N, Gougerot-Pocidalo MA, Aillet F, et al. Redox status of cells influences constitutive or induced NF-kappa B translocation and HIV long terminal repeat activity in human T and monocytic cell lines. J Immunol. 1992;149:3386–93.
Palamara AT, Perno CF, Aquaro S, et al. Glutathione inhibits HIV replication by acting at late stages of the virus life cycle. AIDS Res Hum Retroviruses. 1996;12:1537–41.
Fuchs J, Oelke N, Imhof M, et al. Multiparameter analysis of clastogenic factors, pro-oxidant cytokines, and inflammatory markers in HIV-1-infected patients with asymptomatic disease, opportunistic infections, and malignancies. Mol Med. 1998;4:333–43.
Driscoll KE. TNFalpha and MIP-2: role in particle-induced inflammation and regulation by oxidative stress. Toxicol Lett. 2000;112–113:177–83.
Aguilera G, Colin-Gonzalez AL, Rangel-Lopez E, et al. Redox signaling, neuroinflammation, and neurodegeneration. Antioxid Redox Signal. 2018;28:1626–51.
Roc AC, Ances BM, Chawla S, et al. Detection of human immunodeficiency virus induced inflammation and oxidative stress in lenticular nuclei with magnetic resonance spectroscopy despite antiretroviral therapy. Arch Neurol. 2007;64:1249–57.
Bandaru VV, McArthur JC, Sacktor N, et al. Associative and predictive biomarkers of dementia in HIV-1-infected patients. Neurology. 2007;68:1481–7.
Eden A, Price RW, Spudich S, et al. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J Infect Dis. 2007;196:1779–83.
Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18.
Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol. 2015;36:22–30.
Zevin AS, McKinnon L, Burgener A, et al. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS. 2016;11:182–90.
Mak G, Zaunders JJ, Bailey M, et al. Preservation of gastrointestinal mucosal barrier function and microbiome in patients with controlled HIV infection. Front Immunol. 2021;12:688886.
• Jiang W, Luo Z, Stephenson S, et al. Cerebrospinal fluid and plasma lipopolysaccharide levels in human immunodeficiency virus type 1 infection and associations with inflammation, blood-brain barrier permeability, and neuronal injury. J Infect Dis. 2021;223:1612–1620. A study that assesses both plasma and CSF LPS in viremic PLWH, and demonstrates the link between plasma LPS, increased blood-brain barrier permeability, and neuroinflammation.
Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13.
Valcour VG, Ananworanich J, Agsalda M, et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS ONE. 2013;8:e70164.
Rich S, Klann E, Bryant V, et al. A review of potential microbiome-gut-brain axis mediated neurocognitive conditions in persons living with HIV. Brain Behav Immun Health. 2020;9:100168.
Ceccarelli G, Brenchley JM, Cavallari EN, et al. Impact of high-dose multi-strain probiotic supplementation on neurocognitive performance and central nervous system immune activation of HIV-1 infected individuals. Nutrients. 2017;9(11):1269. https://doi.org/10.3390/nu9111269.
Ceccarelli G, Fratino M, Selvaggi C, et al. A pilot study on the effects of probiotic supplementation on neuropsychological performance and microRNA-29a-c levels in antiretroviral-treated HIV-1-infected patients. Brain Behav. 2017;7:e00756.
•• Angelovich TA, Churchill MJ, Wright EJ, et al. New potential axes of HIV neuropathogenesis with relevance to biomarkers and treatment. In: Cysique, L.A., Rourke, S.B. (eds) Neurocognitive Complications of HIV-Infection. Current Topics in Behavioral Neurosciences, vol 50. Springer, Cham. https://doi.org/10.1007/7854_2019_126. A comprehensive update on HIV neuropathogenesis, relevant biomarkers, and treatment priorities.
Ambrosius B, Gold R, Chan A, et al. Antineuroinflammatory drugs in HIV-associated neurocognitive disorders as potential therapy. Neurol Neuroimmunol Neuroinflamm. 2019;6:e551.
Joshi G, Johnson JA. The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discov. 2012;7:218–29.
Seu L, Burt TD, Witte JS, et al. Variations in the heme oxygenase-1 microsatellite polymorphism are associated with plasma CD14 and viral load in HIV-infected African-Americans. Genes Immun. 2012;13:258–67.
Chen M, Zhou L, Ding H, et al. Short (GT) ( n ) repeats in heme oxygenase-1 gene promoter are associated with lower risk of coronary heart disease in subjects with high levels of oxidative stress. Cell Stress Chaperones. 2012;17:329–38.
Gill AJ, Garza R, Ambegaokar SS, et al. Heme oxygenase-1 promoter region (GT)n polymorphism associates with increased neuroimmune activation and risk for encephalitis in HIV infection. J Neuroinflamm. 2018;15:70.
Gill AJ, Kolson DL. Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in HAND persistence. Curr HIV/AIDS Rep. 2014;11:325–35.
Gill AJ, Kovacsics CE, Cross SA, et al. Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. J Clin Invest. 2014;124:4459–72.
Kovacsics CE, Gill AJ, Ambegaokar SS, et al. Degradation of heme oxygenase-1 by the immunoproteasome in astrocytes: a potential interferon-gamma-dependent mechanism contributing to HIV neuropathogenesis. Glia. 2017;65:1264–77.
• Garza R, Gill AJ, Bastien BL, et al. Heme oxygenase-1 promoter (GT) n polymorphism associates with HIV neurocognitive impairment. Neurol Neuroimmunol Neuroinflamm. 2020;7(3):e710. https://doi.org/10.1212/NXI.0000000000000710. Using the CHARTER cohort, investigators demonstrated a significant risk-reduction for HIV-NCI in African-Americans with short (GT)n dinucleotide repeats in the HMOX1 promoter.
Gill AJ, Kolson DL. Dimethyl fumarate modulation of immune and antioxidant responses: application to HIV therapy. Crit Rev Immunol. 2013;33:307–59.
Deeks ED. Dimethyl fumarate: a review in relapsing-remitting MS. Drugs. 2016;76:243–54.
Cross SA, Cook DR, Chi AW, et al. Dimethyl fumarate, an immune modulator and inducer of the antioxidant response, suppresses HIV replication and macrophage-mediated neurotoxicity: a novel candidate for HIV neuroprotection. J Immunol. 2011;187:5015–25.
Ambrosius B, Faissner S, Guse K, et al. Teriflunomide and monomethylfumarate target HIV-induced neuroinflammation and neurotoxicity. J Neuroinflamm. 2017;14:51.
•• Kornberg MD, Bhargava P, Kim PM, et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science. 2018; 360(6387):449–453. https://doi.org/10.1126/science.aan4665. This study identifies a potential immune-modulating neuroprotective mechanism of action of dimethylfumarate in the brain (switching from aerobic to anaerobic glycolysis), through studies of treated multiple sclerosis patients and animal models.
• Garcia-Mesa Y, Xu HE, Vance P, et al. Dimethyl fumarate, an approved multiple sclerosis treatment, reduces brain oxidative stress in SIV-infected rhesus macaques: potential therapeutic repurposing for HIV neuroprotection. Antioxidants 2021;10(3):416. https://doi.org/10.3390/antiox10030416. A pilot study of the FDA-approved drug, dimethyl fumarate, in SIV-infected, immune-deficient macques that demonstrated reduced DNA and protein oxidation in multiple brain regions during chronic oral administration.
• Robledinos-Anton N, Fernandez-Gines R, Manda G, et al. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev. 2019;2019:9372182. A very useful introductory review of the topic of Nrf2 activators as therapeutics.
Mansouri A, Reiner Z, Ruscica M, et al. Antioxidant effects of statins by modulating Nrf2 and Nrf2/HO-1 signaling in different diseases. J Clin Med. 2022;11(5):1313. https://doi.org/10.3390/jcm11051313.
Ganesan A, Crum-Cianflone N, Higgins J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203:756–64.
Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58:588–95.
Power MC, Weuve J, Sharrett AR, et al. Statins, cognition, and dementia-systematic review and methodological commentary. Nat Rev Neurol. 2015;11:220–9.
Williams TE, Holdsworth KP, Nicholas JM, et al. Assessing neurofilaments as biomarkers of neuroprotection in progressive multiple sclerosis: from the MS-STAT randomized controlled trial. Neurol Neuroimmunol Neuroinflamm. 2022;9(2):e1130. https://doi.org/10.1212/NXI.0000000000001130.
• Stefanou MI, Palaiodimou L, Katsanos AH, et al. The effects of HMG-CoA reductase inhibitors on disease activity in multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. 2022;58:103395. Failure of statins to demonstrate neurprotective effects in multiple sclerosis by neuroimaging and clinical criteria is comprehensively covered.
Schifitto G, Navia BA, Yiannoutsos CT, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21:1877–86.
Zhao Y, Navia BA, Marra CM, et al. Memantine for AIDS dementia complex: open-label report of ACTG 301. HIV Clin Trials. 2010;11:59–67.
Magyar K, Szende B. (-)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicology. 2004;25:233–42.
Schifitto G, Yiannoutsos CT, Ernst T, et al. Selegiline and oxidative stress in HIV-associated cognitive impairment. Neurology. 2009;73:1975–81.
Schifitto G, Zhang J, Evans SR, et al. A multicenter trial of selegiline transdermal system for HIV-associated cognitive impairment. Neurology. 2007;69:1314–21.
Tong N, Sanchez JF, Maggirwar SB, et al. Activation of glycogen synthase kinase 3 beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur J Neurosci. 2001;13:1913–22.
Dou H, Birusingh K, Faraci J, et al. Neuroprotective activities of sodium valproate in a murine model of human immunodeficiency virus-1 encephalitis. J Neurosci. 2003;23:9162–70.
Everall IP, Bell C, Mallory M, et al. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501.
Letendre SL, Woods SP, Ellis RJ, et al. Lithium improves HIV-associated neurocognitive impairment. AIDS. 2006;20:1885–8.
Schifitto G, Zhong J, Gill D, et al. Lithium therapy for human immunodeficiency virus type 1-associated neurocognitive impairment. J Neurovirol. 2009;15:176–86.
Decloedt EH, Freeman C, Howells F, et al. Moderate to severe HIV-associated neurocognitive impairment: a randomized placebo-controlled trial of lithium. Medicine (Baltimore). 2016;95:e5401.
Cysique LA, Maruff P, Brew BJ. Valproic acid is associated with cognitive decline in HIV-infected individuals: a clinical observational study. BMC Neurol. 2006;6:42.
Schifitto G, Peterson DR, Zhong J, et al. Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report. Neurology. 2006;66:919–21.
•• Medeiros GC, Smith FA, Trivedi MH, et al. Depressive disorders in HIV/AIDS: a clinically focused narrative review. Harv Rev Psychiatry. 2020;28:146–158. A comprehensive, yet digestible guide for clinicians evaluating and treating depressive disorders in PLWH.
Eshun-Wilson I, Siegfried N, Akena DH, et al. Antidepressants for depression in adults with HIV infection. Cochrane Database Syst Rev. 2018;1:CD008525.
• Stewart JC, Polanka BM, So-Armah KA, et al. Associations of total, cognitive/affective, and somatic depressive symptoms and antidepressant use with cardiovascular disease-relevant biomarkers in HIV: veterans aging cohort study. Psychosom Med. 2020;82:461–470. An important and comprehensive review of more than 2300 veterans linking cardiovascular disease favorable and unfavorable risk modulation with antidepressant use. The VACS cohort is revealing many important disease associations.
Ances BM, Letendre SL, Alexander T, et al. Role of psychiatric medications as adjunct therapy in the treatment of HIV associated neurocognitive disorders. Int Rev Psychiatry. 2008;20:89–93.
Letendre SL, Marquie-Beck J, Ellis RJ, et al. The role of cohort studies in drug development: clinical evidence of antiviral activity of serotonin reuptake inhibitors and HMG-CoA reductase inhibitors in the central nervous system. J Neuroimmune Pharmacol. 2007;2:120–7.
Sacktor N, Skolasky RL, Moxley R, et al. Paroxetine and fluconazole therapy for HIV-associated neurocognitive impairment: results from a double-blind, placebo-controlled trial. J Neurovirol. 2018;24:16–27.
Ojagbemi A. HIV associated neurocognitive disorders subsidence through citalopram addition in anti-retroviral therapy (HANDS-CARE): a concept note. Front Neurol. 2021;12:658705.
• McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet. 2021;398:1184–1194. A comprehensive, informative review of S1PR modulators that discusses potential uses of this class of drugs in systemic and CNS inflammatory and immune-mediated diseases.
• Pino M, Paganini S, Deleage C, et al. Fingolimod retains cytolytic T cells and limits T follicular helper cell infection in lymphoid sites of SIV persistence. PLoS Pathog. 2019;15:e1008081. A novel study of an FDA-approved inhibitor of immune cell trafficking for targeting lymphoid tissues, with implications for HIV reservoir reduction studies.
Kister I, Patskovsky Y, Curtin R, et al. Cellular and humoral immunity to SARS-CoV-2 infection in multiple sclerosis patients on ocrelizumab and other disease-modifying therapies: a multi-ethnic observational study. Ann Neurol. 2022;91(6):782–95. https://doi.org/10.1002/ana.26346.
Haanstra KG, Hofman SO, Lopes Estevao DM, et al. Antagonizing the alpha4beta1 integrin, but not alpha4beta7, inhibits leukocytic infiltration of the central nervous system in rhesus monkey experimental autoimmune encephalomyelitis. J Immunol. 2013;190:1961–73.
De Kleijn KMA, Martens GJM. Molecular effects of FDA-approved multiple sclerosis drugs on glial cells and neurons of the central nervous system. Int J Mol Sci. 2020;21(12):4229. https://doi.org/10.3390/ijms21124229.
Campbell JH, Ratai EM, Autissier P, et al. Anti-alpha4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014;10:e1004533.
• Gaddam M, Singh A, Jain N, et al. A comprehensive review of intranasal insulin and its effect on the cognitive function of diabetics. Cureus. 2021;13:e17219. Highly informative review of beneficial effects of intranasal insulin on cognitive performance in diabetic patients that supports a rationale for further testing in other disorders.
• Craft S, Raman R, Chow TW, et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and Alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 2020;77:1099–1109. First multisite phase 2/3study of intranasal insulin for cognitive deficits in MCI or Alzheimer’s disease showed no benefit over a 12-month period. It did confirm effective delivery approach and patient compliance.
Monroe AK, Zhang L, Jacobson LP, et al. The association between physical activity and cognition in men with and without HIV infection. HIV Med. 2017;18:555–63.
• Dufour CA, Marquine MJ, Fazeli PL, et al. A longitudinal analysis of the impact of physical activity on neurocognitive functioning among HIV-infected adults. AIDS Behav. 2018;22:1562–1572. An important study (n=235 PLWH, 56 HIV-negative controls) demonstrating the consistent physical activity (> 50% of study visits) associates with better preservation of neurocognitive functioning in both PLWH and HIV-negative controls.
• Vance DE, Fazeli PL, Cheatwood J, et al. Targeting HIV-related neurocognitive impairments with cognitive training strategies: insights from the cognitive aging literature. Curr Top Behav Neurosci. 2021;50:503–515. An informative study for all clinicians and researchers interested in cognitive outcomes in PLWH.
Vance DE, Fazeli PL, Cheatwood J, et al. Computerized cognitive training for the neurocognitive complications of HIV infection: a systematic review. J Assoc Nurses AIDS Care. 2019;30:51–72.
Erlandson KM, Kitch D, Wester CW, et al. The impact of statin and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy on cognitive function in adults with human immunodeficiency virus infection. Clin Infect Dis. 2017;65:2042–9.
Tarumi T, Tomoto T, Repshas J, et al. Midlife aerobic exercise and brain structural integrity: associations with age and cardiorespiratory fitness. Neuroimage. 2021;225:117512.
Dickens AM, Anthony DC, Deutsch R, et al. Cerebrospinal fluid metabolomics implicate bioenergetic adaptation as a neural mechanism regulating shifts in cognitive states of HIV-infected patients. AIDS. 2015;29:559–69.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Central Nervous System and Cognition
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kolson, D.L. Developments in Neuroprotection for HIV-Associated Neurocognitive Disorders (HAND). Curr HIV/AIDS Rep 19, 344–357 (2022). https://doi.org/10.1007/s11904-022-00612-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-022-00612-2