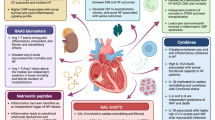
Abstract
Purpose of Review
HIV is an independent risk factor for heart
failure (HF). Cardiac imaging studies in people with HIV (PWH) have identified myocardial pathologies, namely fibrosis and steatosis, that likely contribute to the higher risk of HF. In this review, we survey existing epidemiological, clinical, and mechanistic literature to identify potential pathways that may contribute to the burden of myocardial fibrosis and steatosis among PWH.
Recent Findings
Multiple cohort studies over the past 20 years have demonstrated a roughly 2-fold higher risk of incident HF in PWH, as well as a disproportionate burden of myocardial fibrosis and steatosis in PWH without HF. Both myocardial fibrosis and steatosis are known contributors to HF in adults without HIV. Pathways involving the NLRP3 inflammasome, TGF-β1, and adipocyte dysfunction are known to play a crucial role in the development of myocardial fibrosis and steatosis. Upregulation of these pathways in HIV due to direct effects of viral proteins, persistent immune dysregulation, gut epithelial breakdown and dysbiosis, and toxicities from antiretroviral therapy may contribute to myocardial dysfunction in HIV. Understanding these pathways may lead to more precise diagnostic and therapeutic targets to curb HF in PWH.
Summary
During the past three decades, observational and mechanistic studies have provided important insights into risk factors and pathways that may contribute to the increased HF risk in PWH. Future work is needed to characterize these pathways more precisely in mechanistic studies of PWH, with the goal of ultimately deriving valuable targets for prevention, early diagnosis, and treatment of HF in PWH.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cohen IS, Anderson DW, Virmani R, Reen BM, Macher AM, Sennesh J, et al. Congestive cardiomyopathy in association with the acquired immunodeficiency syndrome. N Engl J Med. 1986;315:628–30.
Calabrese LH, Proffitt MR, Yen-Lieberman B, Hobbs RE, Ratliff NB. Congestive cardiomyopathy and illness related to the acquired immunodeficiency syndrome (AIDS) associated with isolation of retrovirus from myocardium. Ann Intern Med. 1987;107:691–2.
Acierno LJ. Cardiac complications in acquired immunodeficiency syndrome (AIDS): a review. J Am Coll Cardiol. 1989;13:1144–54.
Herskowitz A, Willoughby SB, Baughman KL, Schulman SP, Bartlett JD. Cardiomyopathy associated with antiretroviral therapy in patients with HIV infection: a report of six cases. Ann Intern Med. 1992;116:311–3.
Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS. 2016;11:417–23.
Lederman MM, Funderburg NT, Sekaly RP, Klatt NR, Hunt PW. Residual immune dysregulation syndrome in treated HIV infection. Adv Immunol. 2013;119:51–83.
Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res. 2015;116:1254–68.
Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol. 2020;17:269–85.
Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail. 2017;19:1379–89.
Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–43.
Freiberg MS, Chang CH, Skanderson M, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2:536–46 This study extracted left ventricular ejection fractions from the electronic health records to determine the association of HIV infection with different HF subtypes, specifically HFrEF, HFpEF, and HFmrEF.
Womack JA, Chang CC, So-Armah KA, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3:e001035.
Feinstein MJ, Steverson AB, Ning H, et al. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. J Am Heart Assoc. 2018;7:e009985 This is the only study to date to have evaluated the association of HIV infection with HF, where the HF diagnsoses were underwent physicain adjudication.
Yen YF, Ko MC, Yen MY, Hu BS, Wang TH, Chuang PH, et al. Human immunodeficiency virus increases the risk of incident heart failure. J Acquir Immune Defic Syndr. 2019;80:255–63.
Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. 2020;71:1379–89.
El-Sadr WM, Lundgren J, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96.
Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807.
Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. Jama. 2018;320:379–96.
Erqou S, Lodebo BT, Masri A, Altibi AM, Echouffo-Tcheugui JB, Dzudie A, et al. Cardiac dysfunction among people living with HIV: a systematic review and meta-analysis. JACC Heart Fail. 2019;7:98–108.
Sinha A, Mystakelis H, Rivera AS, et al. Association of low CD4/CD8 ratio with adverse cardiac mechanics in lymphopenic HIV-infected adults. J Acquir Immune Defic Syndr. 2020;85:e73-e76.
Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128:814–22 This study used MRI/MRS to demonstrate that HIV patients without clinical cardiovascular disease had significantly greater myocardial fibrosis and steatosis than uninfected controls.
Thiara DK, Liu CY, Raman F, et al. Abnormal myocardial function is related to myocardial Steatosis and diffuse myocardial fibrosis in HIV-infected adults. J Infect Dis. 2015;212:1544–51 This study used MRI/MRS to further corroborate that HIV patients had a higher burden of myocardial fibrosis and steatosis compared with uninfected controls and that LV function was negatively associated with inflammatory markers.
Feinstein MJ, Mitter SS, Yadlapati A, Achenbach CJ, Palella FJ Jr, Engel Gonzalez P, et al. HIV-related myocardial vulnerability to infarction and coronary artery disease. J Am Coll Cardiol. 2016;68:2026–7.
Nelson MD, Szczepaniak LS, LaBounty TM, et al. Cardiac steatosis and left ventricular dysfunction in HIV-infected patients treated with highly active antiretroviral therapy. JACC Cardiovasc Imaging. 2014;7:1175–7.
González A, Schelbert EB, Díez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71:1696–706.
Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26.
Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210.
Sun Y, Weber KT. Angiotensin converting enzyme and myofibroblasts during tissue repair in the rat heart. J Mol Cell Cardiol. 1996;28:851–8.
Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28.
Schelbert EB, Piehler KM, Zareba KM, Moon JC, Ugander M, Messroghli DR, et al. Myocardial fibrosis quantified by extracellular volume is associated with subsequent hospitalization for heart failure, death, or both across the Spectrum of ejection fraction and heart failure stage. J Am Heart Assoc. 2015;4:e002613.
Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, et al. Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA Cardiol. 2017;2:995–1006.
Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discovery. 2020;6:36.
Mullis C, Swartz TH. NLRP3 Inflammasome signaling as a link between HIV-1 infection and atherosclerotic cardiovascular disease. Front Cardiovasc Med. 2020;7:95.
Bandera A, Masetti M, Fabbiani M, Biasin M, Muscatello A, Squillace N, et al. The NLRP3 Inflammasome is upregulated in HIV-infected antiretroviral therapy-treated individuals with defective immune recovery. Front Immunol. 2018;9:214.
Song J, Jiao Y, Zhang T, Zhang Y, Huang X, Li H, et al. Longitudinal changes in plasma Caspase-1 and Caspase-3 during the first 2 years of HIV-1 infection in CD4Low and CD4High patient groups. PLoS One. 2015;10:e0121011.
Chattergoon MA, Latanich R, Quinn J, et al. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014;10:e1004082 This in vitro study demonstrated that HIV could activate the NLRP3 inflammasome in monocytes and macrophages via an infection-independent process.
Guo H, Gao J, Taxman DJ, Ting JP, Su L. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem. 2014;289:21716–26.
Doitsh G, Galloway NL, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–14.
Galloway NL, Doitsh G, Monroe KM, et al. Cell-to-cell transmission of HIV-1 is required to trigger pyroptotic death of lymphoid-tissue-derived CD4 T cells. Cell Rep. 2015;12:1555–63.
Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis. 2014;210:1228–38.
Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254:54–64.
Assimakopoulos SF, Dimitropoulou D, Marangos M, Gogos CA. Intestinal barrier dysfunction in HIV infection: pathophysiology, clinical implications and potential therapies. Infection. 2014;42:951–9.
Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–45.
Ribeiro A, Heimesaat MM, Bereswill S. Changes of the intestinal microbiome-host homeostasis in HIV-infected individuals - a focus on the bacterial gut microbiome. Eur J Microbiol Immunol (Bp). 2017;7:158–67.
Vujkovic-Cvijin I, Somsouk M. HIV and the gut microbiota: composition, consequences, and avenues for amelioration. Curr HIV/AIDS Rep. 2019;16:204–13.
Colaco NA, Ma Y, Scherzer R, et al. Abstract 15318: transmethylamine-N-oxide (TMAO) is associated with diffuse cardiac fibrosis in persons living with HIV. Circulation. 2019;140:A15318-A15318 This study showed a strong association between TMAO, a metabolite produced by gut bacteria, and myocardial fibrosis in HIV, thus providing a link between gut microbiota dysbiosis and heart failure in this population.
Li X, Geng J, Zhao J, Ni Q, Zhao C, Zheng Y, et al. Trimethylamine N-oxide exacerbates cardiac fibrosis via activating the NLRP3 inflammasome. Front Physiol. 2019;10:866.
Hsue PY, Li D, Ma Y, et al. IL-1β inhibition reduces atherosclerotic inflammation in HIV infection. J Am Coll Cardiol. 2018;72:2809–11 This study showed that IL-1β inhibition reduced vascular inflammation in HIV.
Allen JB, Wong HL, Guyre PM, Simon GL, Wahl SM. Association of circulating receptor Fc gamma RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-beta. J Clin Invest. 1991;87:1773–9.
Reinhold D, Wrenger S, Kähne T, Ansorge S. HIV-1 Tat: immunosuppression via TGF-beta1 induction. Immunol Today. 1999;20:384–5.
Hu R, Oyaizu N, Than S, Kalyanaraman VS, Wang XP, Pahwa S. HIV-1 gp160 induces transforming growth factor-beta production in human PBMC. Clin Immunol Immunopathol. 1996;80:283–9.
Laurence J, Elhadad S, Robison T, Terry H, Varshney R, Woolington S, et al. HIV protease inhibitor-induced cardiac dysfunction and fibrosis is mediated by platelet-derived TGF-β1 and can be suppressed by exogenous carbon monoxide. PLoS One. 2017;12:e0187185.
Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62.
Phetsouphanh C, Xu Y, Zaunders J. CD4 T cells mediate both positive and negative regulation of the immune response to HIV infection: complex role of T follicular helper cells and regulatory T cells in pathogenesis. Front Immunol. 2015;5:681.
Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–17.
Maina EK, Abana CZ, Bukusi EA, Sedegah M, Lartey M, Ampofo WK. Plasma concentrations of transforming growth factor beta 1 in non-progressive HIV-1 infection correlates with markers of disease progression. Cytokine. 2016;81:109–16.
Theron AJ, Anderson R, Rossouw TM, Steel HC. The role of transforming growth factor beta-1 in the progression of HIV/AIDS and development of non-AIDS-defining fibrotic disorders. Front Immunol. 2017;8:1461.
Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care. 2011;34:S371–9.
Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–22.
Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9.
Atkinson LL, Kozak R, Kelly SE, Onay Besikci A, Russell JC, Lopaschuk GD. Potential mechanisms and consequences of cardiac triacylglycerol accumulation in insulin-resistant rats. Am J Physiol Endocrinol Metab. 2003;284:E923–30.
McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–5.
Rijzewijk LJ, van der Meer RW, Smit JW, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52:1793–9.
Korosoglou G, Humpert PM, Ahrens J, Oikonomou D, Osman NF, Gitsioudis G, et al. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging. 2012;35:804–11.
Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LEJ, et al. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol. 2016;310:H14–9.
Tsiodras S, Mantzoros C, Hammer S, Samore M. Effects of protease inhibitors on hyperglycemia, hyperlipidemia, and lipodystrophy: a 5-year cohort study. Arch Intern Med. 2000;160:2050–6.
Carr A, Samaras K, Thorisdottir A, Kaufmann GR, Chisholm DJ, Cooper DA. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093–9.
Caron M, Auclairt M, Vissian A, Vigouroux C, Capeau J. Contribution of mitochondrial dysfunction and oxidative stress to cellular premature senescence induced by antiretroviral thymidine analogues. Antivir Ther. 2008;13:27–38.
McComsey GA, Moser C, Currier J, et al. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis. 2016;62:853–62.
Godfrey C, Bremer A, Alba D, Apovian C, Koethe JR, Koliwad S, et al. Obesity and fat metabolism in human immunodeficiency virus–infected individuals: immunopathogenic mechanisms and clinical implications. J Infect Dis. 2019;220:420–31.
Mori MA, Thomou T, Boucher J, Lee KY, Lallukka S, Kim JK, et al. Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J Clin Invest. 2014;124:3339–51.
Torriani M, Srinivasa S, Fitch KV, Thomou T, Wong K, Petrow E, et al. Dysfunctional subcutaneous fat with reduced dicer and brown adipose tissue gene expression in HIV-infected patients. J Clin Endocrinol Metab. 2016;101:1225–34.
Koethe JR, McDonnell W, Kennedy A, et al. Adipose Tissue is Enriched for Activated and Late-Differentiated CD8+ T Cells and Shows Distinct CD8+ Receptor Usage, Compared With Blood in HIV-Infected Persons. J Acquir Immune Defic Syndr. 2018;77:e14–21 This small study showed that adipose tissue in treated adults with HIV had a higher percentage of activated and late-differentiated CD8 T cells compared with peripheral blood.
Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–20.
Couturier J, Suliburk JW, Brown JM, Luke DJ, Agarwal N, Yu X, et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. Aids. 2015;29:667–74.
Damouche A, Pourcher G, Pourcher V, et al. High proportion of PD-1-expressing CD4(+) T cells in adipose tissue constitutes an immunomodulatory microenvironment that may support HIV persistence. Eur J Immunol. 2017;47:2113–23 In HIV-infected adults on ART, there was high PD-1 expression on adipose-tissue-resident CD4 T cells, which inhibits immune activation and contributes to viral persistence.
Palmer CS, Anzinger JJ, Zhou J, Gouillou M, Landay A, Jaworowski A, et al. Glucose transporter 1-expressing proinflammatory monocytes are elevated in combination antiretroviral therapy-treated and untreated HIV+ subjects. J Immunol. 2014;193:5595–603.
Galván-Peña S, O'Neill LA. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420.
Kosmiski LA, Bacchetti P, Kotler DP, Heymsfield SB, Lewis CE, Shlipak MG, et al. Relationship of fat distribution with adipokines in human immunodeficiency virus infection. J Clin Endocrinol Metab. 2008;93:216–24.
Neilan TG, Nguyen K-L, Zaha VG, et al. Myocardial steatosis among antiretroviral therapy–treated people with human immunodeficiency virus participating in the REPRIEVE Trial. J Infect Dis. 2020;222:S63–9 This was an ancillary study from the REPRIEVE trial comprised of individuals who underwent MRI/MRS and demonstrated increased intramyocardial triglyceride content in individuals with HIV, which was associated with immune dysfunction.
Prevention. CfDCa. Supplemental Report: Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data United States and 6 Dependent Areas. 2018;24:3.
Prasada S, Rivera A, Nishtala A, Pawlowski AE, Sinha A, Bundy JD, et al. Differential associations of chronic inflammatory diseases with incident heart failure. JACC Heart Fail. 2020;8:489–98.
Mann DL. Incident heart failure in chronic inflammatory diseases: is it time to rethink stage a heart failure? JACC Heart Fail. 2020;8:499–500.
Sivanandham R, Kleinman AJ, Sette P, Brocca-Cofano E, Kilapandal Venkatraman SM, Policicchio BB, et al. Nonhuman primate testing of the impact of different Treg depletion strategies on reactivation and clearance of latent simian immunodeficiency virus. J Virol. 2020;94(19):e00533-20.
Ponte R, Mehraj V, Ghali P, Couëdel-Courteille A, Cheynier R, Routy J-P. Reversing gut damage in HIV infection: using non-human primate models to instruct clinical research. EBioMedicine. 2016;4:40–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on HIV Pathogenesis and Treatment
Rights and permissions
About this article
Cite this article
Sinha, A., Feinstein, M.J. Immune Dysregulation in Myocardial Fibrosis, Steatosis, and Heart Failure: Current Insights from HIV and the General Population. Curr HIV/AIDS Rep 18, 63–72 (2021). https://doi.org/10.1007/s11904-020-00536-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11904-020-00536-9