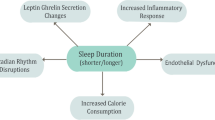
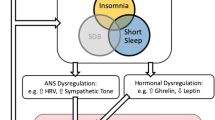
Abstract
Purpose of Review
We discuss the relationship between sleep and circadian factors with cardiovascular disease (CVD) risk, including physiologic, behavioral, and psychological mechanisms along this pathway.
Recent Findings
The relationship between short and long sleep duration, as well as insomnia, with CVD risk is well-established. Recent work has highlighted how other sleep factors, such as sleep regularity (i.e., consistency of sleep timing), multidimensional sleep health, and circadian factors like chronotype and social jetlag, relate to CVD risk. Sleep-focused interventions (e.g., cognitive behavioral therapy for insomnia and sleep extension) may be effective to reduce CVD risk and disease burden.
Summary
Sleep is increasingly recognized as an integral component of cardiovascular health. This was underscored by the recent inclusion of sleep duration as a health behavior in the American Heart Association’s Life’s Essential 8 for defining optimal cardiovascular health.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Matricciani L, Bin YS, Lallukka T, Kronholm E, Wake M, Paquet C, et al. Rethinking the sleep-health link. Sleep Health. 2018;4(4):339–48.
Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156(1):168–97.
Panel: CC, Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Journal of Clinical Sleep Medicine. 2015;11(8):931–52.
St-Onge M-P, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–86.
Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56.
Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36.
St-Onge M-P, Campbell A, Aggarwal B, Taylor JL, Spruill TM, RoyChoudhury A. Mild sleep restriction increases 24-hour ambulatory blood pressure in premenopausal women with no indication of mediation by psychological effects. Am Heart J. 2020;223:12–22.
Abdalla M, Schwartz JE, Cornelius T, Chang BP, Alcántara C, Shechter A. Objective short sleep duration and 24-hour blood pressure. International Journal of Cardiology Hypertension. 2020;7: 100062.
Choudhary AK, Dhanvijay AKD, Alam T, Kishanrao SS. Sleep restriction and its influence on blood pressure. Artery Research. 2017;19:42–8.
Robillard R, Lanfranchi PA, Prince F, Filipini D, Carrier J. Sleep deprivation increases blood pressure in healthy normotensive elderly and attenuates the blood pressure response to orthostatic challenge. Sleep. 2011;34(3):335.
•• Covassin N, Bukartyk J, Singh P, Calvin AD, St Louis EK, Somers VK. Effects of experimental sleep restriction on ambulatory and sleep blood pressure in healthy young adults: a randomized crossover study. Hypertension. 2021;78(3):859–70. In a randomized crossover in-patient study, participants underwent 9 days of sleep restriction (4 hours of sleep/night) or control sleep (9 hours/night) while maintaining a weight maintenance diet. Sleep restriction, compared to control, resulted in increased 24-hour mean blood pressure and plasma norepinephrine levels, and attenuated endothelial function. This experimental study helps to more firmly establish the causal relationship between short sleep duration and cardiovascular risk.
Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, McKinley P, Sloan R, et al. Sleep duration and quality in relation to autonomic nervous system measures: the multi-ethnic study of atherosclerosis (MESA). Sleep. 2016;39(11):1927–40.
Makarem N, Alcántara C, Williams N, Bello NA, Abdalla M. Effect of sleep disturbances on blood pressure. Hypertension. 2021;77(4):1036–46.
Grandner MA, Buxton OM, Jackson N, Sands-Lincoln M, Pandey A, Jean-Louis G. Extreme sleep durations and increased C-reactive protein: effects of sex and ethnoracial group. Sleep. 2013;36(5):769–79.
Abdalla M, Sakhuja S, Akinyelure OP, Thomas SJ, Schwartz JE, Lewis CE, et al. The association of actigraphy-assessed sleep duration with sleep blood pressure, nocturnal hypertension, and nondipping blood pressure: the coronary artery risk development in young adults (CARDIA) study. J Hypertens. 2021;39(12):2478–87.
Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11(5):341–60.
Wang C, Bangdiwala SI, Rangarajan S, Lear SA, AlHabib KF, Mohan V, et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. Eur Heart J. 2019;40(20):1620–9.
Fernandez-Mendoza J, Vgontzas AN. Insomnia and its impact on physical and mental health. Curr Psychiatry Rep. 2013;15(12):1–8.
Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64.
• Ge L, Guyatt G, Tian J, Pan B, Chang Y, Chen Y, et al. Insomnia and risk of mortality from all-cause, cardiovascular disease, and cancer: systematic review and meta-analysis of prospective cohort studies. Sleep Med Rev. 2019;48:101215. This is a large systematic review and meta-analysis consisting of 29 prospective cohort studies (n=1,598,628 participants), with a median follow-up of 10.5 years on the relationship between insomnia symptoms and mortality. Findings indicate that the specific insomnia symptoms difficulty falling asleep and non-restorative sleep were associated with increased risk of all-cause mortality (HRs: 1.13 and 1.23, respectively) and CVD mortality (HRs: 1.20 and 1.48, respectively).
Hsu C-Y, Chen Y-T, Chen M-H, Huang C-C, Chiang C-H, Huang P-H, et al. The association between insomnia and increased future cardiovascular events: a nationwide population-based study. Psychosom Med. 2015;77(7):743–51.
Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31.
Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, et al. Insomnia disorder. Nat Rev Dis Primers. 2015;1(1):1–18.
Javaheri S, Redline S. Insomnia and risk of cardiovascular disease. Chest. 2017;152(2):435–44.
Slavish DC, Graham-Engeland JE, Engeland CG, Taylor DJ, Buxton OM. Insomnia symptoms are associated with elevated C-reactive protein in young adults. Psychol Health. 2018;33(11):1396–415.
Lanfranchi PA, Pennestri M-H, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep. 2009;32(6):760–6.
Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–64.
Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28(11):1295–302.
•• Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events: the multi-ethnic study of atherosclerosis. J Am Coll Cardiol. 2020;75(9):991–9. This analysis from the Multi-Ethnic Study of Atherosclerosis demonstrated a prospective association between sleep regularity and CVD risk in n=1,992 US adults with a mean follow-up period of 4.9 years. Sleep regularity, i.e., day-to-day variability in sleep duration and sleep onset timing, was operationalized as the standard deviation in these metrics. Having sleep duration and onset timing standard deviation >120 vs. ≤60 minutes and >90 vs. ≤30 minutes, respectively, was related to >2-fold higher CVD risk. These findings helped establish sleep variability as a risk factor for CVD events, separate from sleep duration and quality.
Makarem N, Zuraikat FM, Aggarwal B, Jelic S, St-Onge M-P. Variability in sleep patterns: an emerging risk factor for hypertension. Curr Hypertens Rep. 2020;22(2):1–10.
Zuraikat FM, Makarem N, Redline S, Aggarwal B, Jelic S, St-Onge M-P. Sleep regularity and cardiometabolic heath: is variability in sleep patterns a risk factor for excess adiposity and glycemic dysregulation? Curr DiabRep. 2020;20(8):1–9.
Huang T, Redline S. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: the multi-ethnic study of atherosclerosis. Diabetes Care. 2019;42(8):1422–9.
Ogilvie RP, Redline S, Bertoni AG, Chen X, Ouyang P, Szklo M, et al. Actigraphy measured sleep indices and adiposity: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep. 2016;39(9):1701–8.
Lunsford-Avery JR, Engelhard MM, Navar AM, Kollins SH. Validation of the sleep regularity index in older adults and associations with cardiometabolic risk. Sci Rep. 2018;8(1):1–11.
Hoopes EK, Berube FR, D’Agata MN, Patterson F, Farquhar WB, Edwards DG, et al. Sleep duration regularity, but not sleep duration, is associated with microvascular function in college students. Sleep. 2021;44(2):zsaa175.
Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–54.
Fernandez-Mendoza J. The insomnia with short sleep duration phenotype: an update on it’s importance for health and prevention. Curr Opin Psychiatry. 2017;30(1):56–63.
Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–7.
Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60(4):929–35.
•• Bertisch SM, Pollock BD, Mittleman MA, Buysse DJ, Bazzano LA, Gottlieb DJ, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep. 2018;41(6):zsy047. Building off the seminal work from Vgontzas and colleagues from the Penn State Cohort, this recent study from the Sleep Heart Health Study further demonstrated the relationship between insomnia with objective short sleep duration with incident CVD risk. In an analysis of over 4,000 participants, short sleep duration combined with insomnia/poor sleep quality (vs. non-insomnia/non-short sleep group) was associated with significantly higher risk of incident CVD (HR: 1.29). These findings emphasize the need to consider both sleep duration and quality/insomnia in tandem as CVD risk factors.
Jarrin DC, Ivers H, Lamy M, Chen IY, Harvey AG, Morin CM. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J Sleep Res. 2018;27(3): e12663.
Miller CB, Bartlett DJ, Mullins AE, Dodds KL, Gordon CJ, Kyle SD, et al. Clusters of insomnia disorder: an exploratory cluster analysis of objective sleep parameters reveals differences in neurocognitive functioning, quantitative EEG, and heart rate variability. Sleep. 2016;39(11):1993–2004.
Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17.
Wallace ML, Yu L, Buysse DJ, Stone KL, Redline S, Smagula SF, et al. Multidimensional sleep health domains in older men and women: an actigraphy factor analysis. Sleep. 2021;44(2):zsaa181.
Chung J, Goodman M, Huang T, Bertisch S, Redline S. Multidimensional sleep health in a diverse, aging adult cohort: concepts, advances, and implications for research and intervention. Sleep Health. 2021;7(6):699–707.
Wallace ML, Buysse DJ, Redline S, Stone KL, Ensrud K, Leng Y, et al. Multidimensional sleep and mortality in older adults: a machine-learning comparison with other risk factors. The Journals of Gerontology: Series A. 2019;74(12):1903–9.
Li X, Xue Q, Wang M, Zhou T, Ma H, Heianza Y, et al. Adherence to a healthy sleep pattern and incident heart failure: a prospective study of 408 802 UK Biobank participants. Circulation. 2021;143(1):97–9.
Makarem N, Alcantara C, Musick S, Quesada O, Chen Z, Tehranifar P. Multidimensional sleep health is associated with cardiometabolic health in US adults. International Journal of Environmental Research and Public Health. 2022. In press.
• Brindle RC, Yu L, Buysse DJ, Hall MH. Empirical derivation of cutoff values for the sleep health metric and its relationship to cardiometabolic morbidity: results from the Midlife in the United States (MIDUS) study. Sleep. 2019;42(9):zsz116. This study empirically derived cut-off values for “good” and “poor” multidimensional sleep health dimensions using self-report and actigraphy-based sleep measures, and examined the relationship with cardiometabolic morbidity. Sleep dimensions included daytime alertness, quality, timing, regularity, efficiency, duration, and a summed overall total sleep health score was computed. In covariate-adjusted analyses, better sleep health was significantly associated with lower odds of cardiometabolic morbidity (odds ratio: 0.901) and hypertension (odds ratio: 0.903).
Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313(21):1315–22.
Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29(5):992–6.
Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol. 1992;70(1):65–8.
Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354(22):2368–74.
Fagard R, Thijs L, Staessen JA, Clement D, De Buyzere M, De Bacquer D. Night–day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23(10):645–53.
Duffy JF, Dijk D-J. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17(1):4–13.
Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–81.
Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011;108(8):980–4.
Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci. 2010;107(47):20541–6.
Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Investig. 2018;128(6):2157–67.
Andreotti F, Davies GJ, Hackett DR, Khan MI, De Bart AC, Aber VR, et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol. 1988;62(9):635–7.
Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, Willich SN, et al. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987;316(24):1514–8.
Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood, The Journal of the American Society of Hematology. 2014;123(4):590–3.
Scheer FA, Michelson AD, Frelinger AL III, Evoniuk H, Kelly EE, McCarthy M, et al. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS ONE. 2011;6(9): e24549.
Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014;26(2):139–54.
Roenneberg T, Pilz LK, Zerbini G, Winnebeck EC. Chronotype and social jetlag: a (self-) critical review. Biology. 2019;8(3):54.
Taylor BJ, Hasler BP. Chronotype and mental health: recent advances. Curr Psychiatry Rep. 2018;20(8):1–10.
Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36(9):2523–9.
Broms U, Pennanen M, Patja K, Ollila H, Korhonen T, Kankaanpää A, et al. Diurnal evening type is associated with current smoking, nicotine dependence and nicotine intake in the population based National FINRISK 2007 Study. Journal of addiction research & therapy. 2012;S2:002.
Zuraikat FM, St-Onge M-P, Makarem N, Boege HL, Xi H, Aggarwal B. Evening chronotype is associated with poorer habitual diet in us women, with dietary energy density mediating a relation of chronotype with cardiovascular health. J Nutr. 2021;151(5):1150–8.
Kanerva N, Kronholm E, Partonen T, Ovaskainen M-L, Kaartinen NE, Konttinen H, et al. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int. 2012;29(7):920–7.
Maukonen M, Kanerva N, Partonen T, Kronholm E, Konttinen H, Wennman H, et al. The associations between chronotype, a healthy diet and obesity. Chronobiol Int. 2016;33(8):972–81.
Taylor BJ, Bowman MA, Brindle A, Hasler BP, Roecklein KA, Krafty RT, et al. Evening chronotype, alcohol use disorder severity, and emotion regulation in college students. Chronobiol Int. 2020;37(12):1725–35.
Makarem N, Paul J, Giardina E-GV, Liao M, Aggarwal B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiology international. 2020;37(5):673–85.
Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int. 2013;30(4):470–7.
Porcheret K, Wald L, Fritschi L, Gerkema M, Gordijn M, Merrrow M, et al. Chronotype and environmental light exposure in a student population. Chronobiol Int. 2018;35(10):1365–74.
Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–43.
Caliandro R, Streng AA, van Kerkhof LW, van der Horst GT, Chaves I. Social jetlag and related risks for human health: a timely review. Nutrients. 2021;13(12):4543.
Abbott SM, Weng J, Reid KJ, Daviglus ML, Gallo LC, Loredo JS, et al. Sleep timing, stability, and BP in the sueno ancillary study of the hispanic community health study/study of latinos. Chest. 2019;155(1):60–8.
Mota MC, Silva CM, Balieiro LCT, Fahmy WM, Crispim CA. Social jetlag and metabolic control in non-communicable chronic diseases: a study addressing different obesity statuses. Sci Rep. 2017;7(1):1–8.
Rutters F, Lemmens SG, Adam TC, Bremmer MA, Elders PJ, Nijpels G, et al. Is social jetlag associated with an adverse endocrine, behavioral, and cardiovascular risk profile? J Biol Rhythms. 2014;29(5):377–83.
McMahon DM, Burch JB, Youngstedt SD, Wirth MD, Hardin JW, Hurley TG, et al. Relationships between chronotype, social jetlag, sleep, obesity and blood pressure in healthy young adults. Chronobiol Int. 2019;36(4):493–509.
Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social jetlag, chronotype, and cardiometabolic risk. J Clin Endocrinol Metab. 2015;100(12):4612–20.
Kervezee L, Shechter A, Boivin DB. Impact of shift work on the circadian timing system and health in women. Sleep Med Clin. 2018;13(3):295–306.
Åkerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med. 2003;53(2):89–94.
Wickwire EM, Geiger-Brown J, Scharf SM, Drake CL. Shift work and shift work sleep disorder: clinical and organizational perspectives. Chest. 2017;151(5):1156–72.
Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, et al. Shift work and vascular events: systematic review and meta-analysis. Bmj. 2012;345.
Wang D, Ruan W, Chen Z, Peng Y, Li W. Shift work and risk of cardiovascular disease morbidity and mortality: a dose–response meta-analysis of cohort studies. Eur J Prev Cardiol. 2018;25(12):1293–302.
Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, et al. Shift work is a risk factor for increased blood pressure in Japanese men: a 14-year historical cohort study. Hypertension. 2008;52(3):581–6.
Kario K, Schwartz JE, Gerin W, Robayo N, Maceo E, Pickering TG. Psychological and physical stress-induced cardiovascular reactivity and diurnal blood pressure variation in women with different work shifts. Hypertens Res. 2002;25(4):543–51.
• Gamboa Madeira S, Reis C, Paiva T, Moreira CS, Nogueira P, Roenneberg T. Social jetlag, a novel predictor for high cardiovascular risk in blue-collar workers following permanent atypical work schedules. J Sleep Res. 2021;30(6):e13380. This study examined two important factors associated with circadian misalignment that may influence CVD outcomes, namely shift work and social jetlag. In a cross-sectional analysis of permanent shift workers, social jetlag was calculated as the absolute difference between the midpoint of sleep on workdays and a sleep-corrected midpoint of sleep on work-free days based on the Munich ChronoType Questionnaire. Cardiovascular risk was defined with the relative risk Systematic COronary Risk Evaluation (SCORE) chart, based on smoking status, systolic blood pressure, and total cholesterol levels. Mean social jetlag in study participants was approximately 2 hours. Each additional hour of social jetlag was associated with >30% increased CVD risk. This study is among the first showing a relationship between social jetlag and cardiovascular risk in a real-life setting in a particularly susceptible population.
Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci. 2016;113(10):E1402–11.
Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer FA. Circadian misalignment increases C-reactive protein and blood pressure in chronic shift workers. J Biol Rhythms. 2017;32(2):154–64.
Grimaldi D, Carter JR, Van Cauter E, Leproult R. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension. 2016;68(1):243–50.
Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, et al. Actigraphy-derived daily rest–activity patterns and body mass index in community-dwelling adults. Sleep. 2017;40(12):zsx168.
Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–92.
Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14(7):1209–30.
Cornelissen G. Cosinor-based rhythmometry. Theor Biol Med Model. 2014;11(1):1–24.
Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16(4):505–18.
Paudel ML, Taylor BC, Ancoli-Israel S, Stone KL, Tranah G, Redline S, et al. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int. 2011;28(3):258–66.
Sohail S, Yu L, Bennett DA, Buchman AS, Lim AS. Irregular 24-hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int. 2015;32(6):802–13.
Hoopes EK, Witman MA, D’Agata MN, Berube FR, Brewer B, Malone SK, et al. Rest-activity rhythms in emerging adults: implications for cardiometabolic health. Chronobiol Int. 2021;38(4):543–56.
Cheng P, Luik AI, Fellman-Couture C, Peterson E, Joseph CL, Tallent G, et al. Efficacy of digital CBT for insomnia to reduce depression across demographic groups: a randomized trial. Psychol Med. 2019;49(3):491–500.
Luik AI, Bostock S, Chisnall L, Kyle SD, Lidbetter N, Baldwin N, et al. Treating depression and anxiety with digital cognitive behavioural therapy for insomnia: a real world NHS evaluation using standardized outcome measures. Behav Cogn Psychother. 2017;45(1):91–6.
Hall MH, Brindle RC, Buysse DJ. Sleep and cardiovascular disease: Emerging opportunities for psychology. Am Psychol. 2018;73(8):994.
Seixas AA, Vallon J, Barnes-Grant A, Butler M, Langford AT, Grandner MA, et al. Mediating effects of body mass index, physical activity, and emotional distress on the relationship between short sleep and cardiovascular disease. Medicine. 2018;97(37).
Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–68.
Gangwisch JE, Malaspina D, Posner K, Babiss LA, Heymsfield SB, Turner JB, et al. Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. Am J Hypertens. 2010;23(1):62–9.
Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204.
Erten Uyumaz B, Feijs L, Hu J. A review of digital cognitive behavioral therapy for insomnia (CBT-I apps): are they designed for engagement? Int J Environ Res Public Health. 2021;18(6):2929.
Zhou F-C, Yang Y, Wang Y-Y, Rao W-W, Zhang S-F, Zeng L-N, et al. Cognitive behavioural therapy for insomnia monotherapy in patients with medical or psychiatric comorbidities: a meta-analysis of randomized controlled trials. Psychiatr Q. 2020;91(4):1209–24.
• Redeker NS, Yaggi HK, Jacoby D, Hollenbeak CS, Breazeale S, Conley S, et al. Cognitive behavioral therapy for insomnia has sustained effects on insomnia, fatigue, and function among people with chronic heart failure and insomnia: the HeartSleep Study. Sleep. 2022;45(1):zsab252. This was an RCT evaluating the effects of CBT-I on insomnia severity, sleep characteristics, and function in patients with heart failure. This study confirmed that administering CBT-I is feasible in these patients and that the intervention results in sustained, and comparatively larger, improvements in insomnia severity, self-reported sleep quality, daytime sleepiness, and functional performance (6-minute walk test) vs. control. These findings have ramifications for treatment considerations in patients with heart failure who commonly experience insomnia.
Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N, et al. Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: a randomized controlled trial. Biol Psychiat. 2015;78(10):721–9.
Redeker NS, Conley S, Anderson G, Cline J, Andrews L, Mohsenin V, et al. Effects of cognitive behavioral therapy for insomnia on sleep, symptoms, stress, and autonomic function among patients with heart failure. Behavioral sleep medicine. 2018.
YANG X-j, ZHANG Y-f, Juan L, LIU Y-z, Ying L, WANG Y-j, et al. The efficacy of internet-based cognitive behavior therapy on blood pressure for comorbid hypertension and insomnia. Medical Journal of Chinese People's Liberation Army. 2017;42(4):331–5.
McGrath ER, Espie CA, Power A, Murphy AW, Newell J, Kelly C, et al. Sleep to lower elevated blood pressure: a randomized controlled trial (SLEPT). Am J Hypertens. 2017;30(3):319–27.
Johann AF, Hertenstein E, Feige B, Akram U, Holub F, Baglioni C, et al. Cognitive behavioural therapy for insomnia does not appear to have a substantial impact on early markers of cardiovascular disease: A preliminary randomized controlled trial. J Sleep Res. 2020;29(5): e13102.
Baron KG, Duffecy J, Reutrakul S, Levenson JC, McFarland MM, Lee S, et al. Behavioral interventions to extend sleep duration: A systematic review and meta-analysis. Sleep Med Rev. 2021;60: 101532.
Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, Mullington JM. Increasing sleep duration to lower beat-to-beat blood pressure: A pilot study. J Sleep Res. 2013;22(3):295–304.
• Baron KG, Duffecy J, Richardson D, Avery E, Rothschild S, Lane J. Technology assisted behavior intervention to extend sleep among adults with short sleep duration and prehypertension/stage 1 hypertension: a randomized pilot feasibility study. J Clin Sleep Med. 2019;15(11):1587–97. This RCT demonstrated the feasibility and preliminary efficacy of a technology-assisted intervention to extend sleep duration in individuals with short sleep duration and high blood pressure. Sleep was tracked objectively with accelerometry. The 6-week intervention consisted of a didactic component and brief telephone coaching to achieve sleep extension goals. The intervention group demonstrated significantly greater improvements in total sleep time and reductions in systolic and diastolic blood pressure compared to the control group.
Stock AA, Lee S, Nahmod NG, Chang A-M. Effects of sleep extension on sleep duration, sleepiness, and blood pressure in college students. Sleep Health. 2020;6(1):32–9.
•• Lloyd-Jones DM, Allen NB, Anderson CA, Black T, Brewer LC, Foraker RE, et al. Life’s Essential 8: Updating and enhancing the american heart association’s construct of cardiovascular health: A presidential advisory from the american heart association. Circulation. 2022:10.1161/CIR.0000000000001078. This publication is an overview of the AHA’s most recent construct of cardiovascular health, Life’s Essential 8. The key role of sleep for CVD prevention has been formally acknowledged in this construct to define optimal cardiovascular health. The other factors include diet, physical activity, smoking, obesity, cholesterol, blood glucose and blood pressure. Evidence and recommendations for the inclusion of sleep health as component of cardiovascular health are provided.
Makarem N, St-Onge M-P, Liao M, Lloyd-Jones DM, Aggarwal B. Association of sleep characteristics with cardiovascular health among women and differences by race/ethnicity and menopausal status: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. Sleep Health. 2019;5(5):501–8.
• Makarem N, Castro-Diehl C, St-Onge MP, Redline S, Shea S, Lloyd-Jones DM, Ning H, Aggarwal B. Redefining cardiovascular health to include sleep: prospective associations with cardiovascular disease in the MESA Sleep Study. J Am Heart Assoc. 2022;11:e025252. https://doi.org/10.1161/JAHA.122.025252. This prospective cohort study of middle-aged to older U.S. adults is the first to demonstrate that the inclusion of sleep as an eighth metric of cardiovascular health, in addition to the health factors and behaviors included in the AHA’s original Life’s Simple 7, predicts CVD risk over and beyond the original metrics. This publication demonstrates that cardiovascular health scores that include an 8th sleep metric based on sleep duration only or multidimensional sleep health both predicted future CVD risk. These findings highlight that assessing sleep duration may represent a feasible approach for evaluating overall sleep health in a clinic or public health setting when comprehensive multidimensional sleep health assessment is not possible.
Funding
JB is supported by a training grant from the National Center for Advancing Translational Sciences TL1-TR001875. NM is supported by NHLBI grant R00HL148511 and American Heart Association Grant #21CDA855050. AS is supported by NHLBI grants R01HL141494, R01HL157341, and R01HL146911.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Psychological Aspects of Cardiovascular Diseases
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belloir, J., Makarem, N. & Shechter, A. Sleep and Circadian Disturbance in Cardiovascular Risk. Curr Cardiol Rep 24, 2097–2107 (2022). https://doi.org/10.1007/s11886-022-01816-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01816-z