Abstract
Purpose of Review
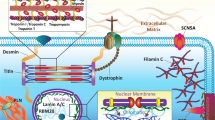
Dilated cardiomyopathy (DCM) is characterized by left ventricular dilation and systolic function and is the most common among all cardiomyopathies. Familial DCM makes up a significant portion of cases, and approximately 40 genes are identified as involved in the pathogenesis of heart failure, each affecting a specific part of cellular mechanisms. The purpose of this review is to summarize recent findings and the current understanding of the most common gene mutations identified associated with DCM.
Recent Findings
Next-generation sequencing is a comprehensive gene analysis technique used to discover more mutation variants and also to learn about the impact of mutations in relationship to clinical presentations. A variety of techniques are utilized to study different gene mutations, such as genotype-phenotype association analysis or whole-exome sequencing, to understand the natural history of diseases. For certain genetic abnormalities, information is helpful in developing potential therapeutic treatment targeting mutations.
Summary
More treatment options are hopeful with the understanding of specific genetic mutations and their pathogenic mechanism. It also suggests the importance of genetic assessment and counseling for family members of an affected patient, in order to provide potential early diagnosis and better clinical management of DCM.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–2.
Mestroni L, Brun F, Spezzacatene A, Sinagra G, Taylor MRG. Genetic causes of dilated cardiomyopathy. Prog Pediatr Cardiol. 2014;37:13–8. https://doi.org/10.1016/j.ppedcard.2014.10.003.
•• Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: A scientific statement from the American Heart Association. Circulation. 2016;134:579–646. Doi: https://doi.org/10.1161/CIR.0000000000000455. A summary of knowledge regarding DCM, emphasizing recent developments in therapeutic approaches for cardiomyopathies.
Bashore TM, Granger CB, Jackson KP, Patel MR. Heart disease. In: Papadakis MA, McPhee SJ, Rabow MW, editors. Current Medical Diagnosis & Treatment 2018. New York: McGraw-Hill; 2018.
•• Bakalakos A, Risatos K, Anastasakis A. Current perspective on the diagnosis and management of dilated cardiomyopathy Beyond heart failure: a Cardiomyopathy Clinic Doctor’s point of view. Hellenic Journal of Cardiology. 2018. https://doi.org/10.1016/j.hjc/2018.05.008 Provides a comprehensive review of DCM diagnosis and management, including new classification concepts and identified genes by the American Heart Association.
Mestroni L, Rocco C, Gregori D, Sinagra G, Lenarda A, Miocic S. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity. J Am Coll Cardiol. 1999;34:181–90. https://doi.org/10.1016/S0735-1097(99)00172-2.
Tabish AM, Azzimato V, Alexiadis A, Buyandelger B, Knöll R. Genetic epidemiology of titin-truncating variants in the etiology of dilated cardiomyopathy. Biophys Rev. 2017;9:207–23. https://doi.org/10.1007/s12551-017-0265-7.
Herman DS, Lam L, Taylor MRG, Wang L, et al. Truncation of titin causing dilated cardiomyopathy. N Engl J Med. 2012;366:619–28. https://doi.org/10.1056/NEJMoa1110186.
Granzier HL, Labeit S. The giant protein titin a major player in myocardial mechanics, signaling and disease. Circ Res. 2004;94:284–95. https://doi.org/10.1161/01.RES.0000117769.88862.F8.
Akinrinade O, Alastalo TP, Koskenvuo JW. Relevance of truncating titin mutations in dilated cardiomyopathy. Clin Genet. 2016;90:49–54. https://doi.org/10.111/cge.12741.
Pérez-Serra A, Toro R, Sarquella-Brugada G, Gonzalo-Calvo D, Cesar S, Carro E, et al. Genetic basis of dilated cardiomyopathy. Int J Cardiol. 2016;224:461–72. https://doi.org/10.1016/j.ijcard.2016.09.068.
• Gigli M, Begay RL, Morea G, Graw SL, Sinagra G, Tylor MRG, et al. A review of the giant protein titin in clinical molecular diagnostic of cardiomyopathies. Front Cardiovasc Med. 2016;3:21. https://doi.org/10.3389/fcvn.2016.00021 Describes TTN genetic mutations in DCM and outlines the importance of detecting mutations clinically.
Begay RL, Graw S, Sinagra G, Merlo M, Slavov D, Gowan K, et al. Role of titin missense variants in dilated cardiomyopathy. J Am Heart Assco. 2015;4:e002645. https://doi.org/10.1161/JAHA.115.002645.
•• Gramlich M, Pane LS, Zhou Q, Chen Z, Mugia M, et al. Antisense-mediated exon skipping: a therapeutic strategy for titin-based dilated cardiomyopathy. EMBO Mol Med. 2015;7:562–76. https://doi.org/10.15252/emmm.201505047 Important discovery of novel therapy highlights the possibility of rescuing genetic deficit by titin in DCM.
• Merlo M, Sinagra G, Carneil E, Slavov D, Zhu X, Barbati G, et al. Poor Prognosis of rare sacromeric gene variants in patients with dilated cardiomyopathy. Clin Transl Sci. 2013;6:424–8. https://doi.org/10.111/cts.12116 Data from the study implies early and aggressive therapy for mutation carriers with DCM.
Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Bio Chem. 1993;268:16321–7.
Tesson F, Saj M, Uvaize MM, Nicolas H, Ploski R, Bilinska Z. Lamin A/C mutations in idlated cardiomyopathy. Cardiology J. 2014;21:331–42. https://doi.org/10.5603/CJ.a2014.0037.
Hershberger RE, Morales A. LMNA-related dilated cardiomyopathy. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews. 1993-2018.
Becane HM, Bonne G, Varnous D, et al. High incidence of sudden death with conduction system and myocardial disease due to lamins A and C gene mutation. Pacing Clin Electrophysiol. 2000;23:1661–6.
Magi S, Lariccia V, Maiolino M, Amoroso S, Gratteri S. Sudden cardiac death: focus on the genetics of channelopathies and cardiomyopathies. J Biomed Sci. 2017;24:56. https://doi.org/10.1186/s12929-017-0364-6.
Pérez-Serra A, Toro R, Campuzano O, Brundaga G. Berene P, Iglesias A, et al. A novel mutation in Lamin A/C causing familial dilated cardiomyopathy associated with sudden cardiac death. J Card Fail 2015;21:217–225. Doi:https://doi.org/10.1016/j.cardfail.2014.12.003.
• Brayson D, Shanahan CM. Current insights into LMNA cardiomyopthies: existing models and missing LINCs. Nucleus. 2017;8:17–33. https://doi.org/10.1080/19491034.2016.1260798 Proposed potential hypotheses to improve our understanding of underlying pathogenesis of LMNA mutations in DCM.
Roncarati R, Anselmi CV, Krawitz P, Lattanzi G, Kodolitsch YV, Perrot A, et al. Doubly heterozygous LMNA and TTN mutations revealed by exome sequencing in a severe form of dilated cardiomyopathy. Eur J Hum Genet. 2013;21:1105–11. https://doi.org/10.1038/ejhg.2013.16.
Zaklyazminskaya E, Dzemeshkevich S. The role of mutations in SCN5A gene in cardiomyopathies. Biochimica et Biophysica Acata – Molecular Cell Research. 1863;2016:1799–805. https://doi.org/10.1016/j.bbamcr.2016.02.014.
McNair WP, Ku L, Taylor MRG, et al. SCN51 mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation. 2004;12:2163–7. https://doi.org/10.1161/01.CIR.0000144458.58660.BB.
Moreau A, Gosselin-Badaroudine P, Chahine M. Gating pore currents, a new pathological mechanism underlying cardiac arrhythmias associated with dilated cardiomyopathy. Channels (Austins). 2015;9:139–44. https://doi.org/10.1080/19936950.2015.1031937.
Moreau A, Mercier A, Gosselin-Badaroudine P, Burger B, Keller GI, Chahine M. Abstract 15933: SCN5A gating pore current causes cardiac arrhythmias associated with dilated cardiomyopathy. Circulation. 2015;132:A15933.
Shen C, Xu L, Han S, Dong Z, Zhao X, Wang S, et al. Novel idiopathic DCM-related SCN5A variants localized in DI-S4 predispose electrical disorders by reducing peak sodium current density. J Med Genet. 2017;54:762–70. https://doi.org/10.1136/jmedgenet-2017-104780.
Young HS, Ceholski DK, Trieber CA. Deception in simplicity: hereditary phopholamban mutations in dilated cardiomyopathy. Biochem Cell Biol. 2015;93:1–7. https://doi.org/10.1139/bcb-2014-0080.
Liu GS, Morales A, Vafiadaki E, Lam CK, Cai WF, Haghighi K, et al. A novel human R25C-phophoblamban mutation is associated with super-inhibition of calcium cycling and ventricular arrhythmia. Cardiovasc Res. 2015;107:164–74. https://doi.org/10.1093/cvr/cvv127.
Sanoudou D, Kolokathis F, Arvanitis D, Al-Shafai K, Krishnamoorthy N, Buchan RJ, et al. Genetic modifiers to the PLN L39X mutation in a patient with DCM and sustained ventricular tachycardia? Glob Cardiol Sci Pract. 2015;2015:29. https://doi.org/10.15339/gcsp.2015.29.
Knezevic T, Myer VD, Gordon J, Tilley DG, Sharp TE III, Wang J, et al. BAG3:a new player in the heart failure paradigm. Heart Fail Rev. 2015;20:423–34. https://doi.org/10.1007/s10741-015-9487-6.
Arimura T, Ishikawa T, Nunoda S, Kawai S, Kimura A. Dilated cardiomyopathy-associated BAG3 mutations impair Z-disc assembly and enhance sensitivity to apoptosis in cardiomyocytes. Hum Mutat. 2011;32:1481–91. https://doi.org/10.1002/humu.21603.
Norton N, Li D, Rideder MJ, Siegfried JD, Rampersaud E, Züchner S, et al. Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy. Am J Hum Genet. 2011;88:273–82. https://doi.org/10.1016/j/ajhg.2011.01.016.
Feldman A, Begay RL, Knezevia T, Myers VD, Slavov DB, Zhu W, et al. Decreased levels of BAG3 in a family with a rare variant and in idiopathic dilated cardiomyopathy. J cell Phsyiol. 2014;229:1697–702. https://doi.org/10.1002/jcp.24615.
Toro R, Pérez-Serra A, Campuzano O, Moncayo-Arlandi J, Allegue C, Iglesias A, et al. Familial dilated cardiomyopathy caused by a novel frameshift in the BAG3 gene. PLoS One. 2015;11:e0158730. https://doi.org/10.1371/journal.pone.0158730.
•• Hershberger RE, Givertz MM, Ho CY, Judge DP, et al. Genetic evaluation of cardiomyopathy – a heart failure society of America practice guideline. J Card Fail. 2018;24:281–302. https://doi.org/10.1016/j.cardfail.2018.03.004 Discusses the most updated procedures and guidelines for genetic testing of cardiomyopathies.
Falk RH, Hershberger RE. The dilated, restrictive, infiltrative cardiomyopathies. In: Mann DL, Zipes DP, Libby P, Bonow RO, editors. Braunwald’s heart disease: a textbook of cardiovascular medicine. Philadelphia: Elsevier/Saunder; 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yiwen Fu and Howard J. Eisen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Heart Failure
Rights and permissions
About this article
Cite this article
Fu, Y., Eisen, H.J. Genetics of Dilated Cardiomyopathy. Curr Cardiol Rep 20, 121 (2018). https://doi.org/10.1007/s11886-018-1061-0
Published:
DOI: https://doi.org/10.1007/s11886-018-1061-0