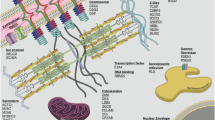
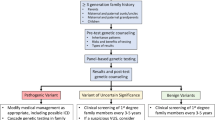
Abstract
Dilated cardiomyopathy (DCM) is one of the most common and clinically heterogeneous forms of cardiomyopathy characterized by a high risk of unfavorable course and outcome. A complex etiology has been shown for DCM. Genetic factors contribute to both familial and sporadic cases. The review summarizes information on the role of genetic factors in the clinical variability of DCM. Much of the data accumulated to date indicates a high genetic heterogeneity of DCM. There are numerous rare pathogenic variants in more than 100 genes that lead to the disease. The type, number, and localization of these genetic variants can affect the clinical course of DCM. Furthermore, common genetic variants are localized in various loci, including genomic regulatory regions and genes of “monogenic forms” of DCM, acting as factors modifying the pathological phenotype.


Similar content being viewed by others
REFERENCES
Richardson, P., McKenna, W., Bristow, M., et al., Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies, Circulation, 1996, vol. 93, no. 5, pp. 841—842. https://doi.org/10.1161/01.cir.93.5.841
Pinto, Y.M., Elliott, P.M., Arbustini, E., et al., Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases, Eur. Heart J., 2016, vol. 37, no. 23, pp. 1850—1858. https://doi.org/10.1093/eurheartj/ehv727
Verdonschot, J.A.J., Hazebroek, M.R., Krapels, I.P.C., et al., Implications of genetic testing in dilated cardiomyopathy, Circ. Genom. Precis. Med., 2020, vol. 13, no. 5, pp. 476—487. https://doi.org/10.1161/circgen.120.003031
McKenna, W.J. and Judge, D.P., Epidemiology of the inherited cardiomyopathies, Nat. Rev. Cardiol., 2021, vol. 18, no. 1, pp. 22—36. https://doi.org/10.1038/s41569-020-0428-2
Lannou, S., Mansencal, N., Couchoud, C., et al., The public health burden of cardiomyopathies: insights from a nationwide inpatient study, J. Clin. Med., 2020, vol. 9. no. 4, p. 920. https://doi.org/10.3390/jcm9040920
McKenna, W.J., Maron, B.J., and Thiene, G., Classification, epidemiology, and global burden of cardiomyopathies, Circ. Res., 2017, vol. 121, no. 7, pp. 722—730. https://doi.org/10.1161/circresaha.117.309711
Chernyavskiy, A.M., Doronin, D.V., Fomichev, A.V., et al., 10-year heart transplantation experience in Novosibirsk, Vestn. Transplantologii Iskusstv. Organov, 2018, vol. 20, no. 1, pp. 23—31.
Cannatà, A., Fabris, E., Merlo, M., et al., Sex differences in the long-term prognosis of dilated cardiomyopathy, Can. J. Cardiol., 2020, vol. 36, no. 1, pp. 37—44. https://doi.org/10.1016/j.cjca.2019.05.031
Werner, N., Nickenig, G., and Sinning, J.M., Complex PCI procedures: challenges for the interventional cardiologist, Clin. Res. Cardiol., 2018, vol. 107, suppl. 2, pp. 64—73. https://doi.org/10.1007/s00392-018-1316-1
Leontyeva, I.V., Problems of modern diagnostics and treatment of dilated cardiomyopathy in children, Ross. Vestn. Perinatol. Pediatr., 2018, vol. 63, no. 2, pp. 7—15. https://doi.org/10.21508/1027-4065-2018-63-2-7-15
Bondue, A., Arbustini, E., Bianco, A., et al., Complex roads from genotype to phenotype in dilated cardiomyopathy: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology, Cardiovasc. Res., 2018, vol. 114, no.10, pp. 1287—1303. https://doi.org/10.1093/cvr/cvy122
Vaykhanskaya, T.G., Sivitskaya, L.N., Kurushko, T.V., et al., Dilated cardiomyopathy: reconceptualization of the problem, Ross. Kardiol. Zh., 2019, vol. 24, no. 4, pp. 35—47. https://doi.org/10.15829/1560-4071-2019-4-35-47
De Bellis, A., De Angelis, G., Fabris, E., et al., Gender-related differences in heart failure: beyond the “one-size-fits-all” paradigm, Heart Failure Rev., 2020, vol. 25, no. 2, pp. 245—255. https://doi.org/10.1007/s10741-019-09824-y
Rosenbaum, A.N., Agre, K.E., and Pereira, N.L., Genetics of dilated cardiomyopathy: practical implications for heart failure management, Nat. Rev. Cardiol., 2020, vol. 17, no. 5, pp. 286—297. https://doi.org/10.1038/s41569-019-0284-0
Jordan, E. and Hershberger, R.E., Considering complexity in the genetic evaluation of dilated cardiomyopathy, Heart, 2021, vol. 107, no. 2, pp. 106—112. https://doi.org/10.1136/heartjnl-2020-316658
Jordan, E., Peterson, L., Ai, T., et al., Evidence-based assessment of genes in dilated cardiomyopathy, Circulation, 2021, vol. 144, no. 1, pp. 7—19. https://doi.org/10.1161/circulationaha.120.053033
Sivasankaran, S., Sharland, G.K., and Simpson, J.M., Dilated cardiomyopathy presenting during fetal life, Cardiol. Young, 2005, vol. 15, no. 4, pp. 409—416. https://doi.org/10.1017/S1047951105000855
Kuo, K., Speranza, R., and Hackmon, R., Fetal dilated cardiomyopathy associated with variants of uncertain significance in MYH7 and DSG2 genes: a case report and review of the literature, J. Obstet. Gynaecol. Can., 2020, vol. 42, no. 9, pp. 1147—1150. https://doi.org/10.1016/j.jogc.2019.11.002
Cohen, J.A. and Almodovar, M.C., Dilated cardiomyopathy in children: moving beyond traditional pharmacologic therapy, Curr. Opin. Cardiol., 2020, vol. 35, no. 1, pp. 52—57. https://doi.org/10.1097/HCO.0000000000000692
Weng, K.P., Lin, C.C., Huang, S.H., and Hsieh, K.S., Idiopathic dilated cardiomyopathy in children: a single medical center’s experience, J. Chin. Med. Assoc., 2005, vol. 68, no. 8, pp. 368—372. https://doi.org/10.1016/S1726-4901(09)70177-7
Halliday, B.P., Gulati, A., Ali, A., et al., Sex- and age-based differences in the natural history and outcome of dilated cardiomyopathy, Eur. J. Heart Failure, 2018, vol. 20, no. 10, pp. 1392—1400. https://doi.org/10.1002/ejhf.1216
Jammal Addin, M.B., Young, D., McCarrison, S., and Hunter, L., Dilated cardiomyopathy in a national paediatric population, Eur. J. Pediatr., 2019, vol. 178, no. 8, pp. 1229—1235. https://doi.org/10.1007/s00431-019-03404-w
Huertas-Quiñones, V.M., Mestra, C.F., Peña-Trujillo, V., et al., Paediatric cardiomyopathies: echocardiographic diagnosis, clinical profile, and demographic characteristics: the experience of a tertiary referral centre for Latin American paediatric cardiology, Cardiol. Young, 2020, vol. 30, no. 4, pp. 462—467. https://doi.org/10.1017/S1047951120000281
Franaszczyk, M., Chmielewski, P., Truszkowska, G., et al., Titin truncating variants in dilated cardiomyopathy—prevalence and genotype—phenotype correlations, PLoS One, 2017, vol. 12, no. 1. e0169007. https://doi.org/10.1371/journal.pone.0169007
Soares, P., Rocha, G., Pissarra, S., et al., Neonatal dilated cardiomyopathy, Rev. Port. Cardiol., 2017, vol. 36, no. 3, pp. 201—214. https://doi.org/10.1016/j.repc.2016.10.007
Mikhailov, V.S., Bukaeva, A.A., Rumyantseva, V.A., et al., Molecular genetic analysis of the TTN gene in children with dilated cardiomyopathy, Klin. Eksp. Khir. Zh. im. Akad. B.V. Petrovskogo, 2018, vol. 6, no. 19, pp. 70—76.
Zaklyazminskaya, E.V., Bukaeva, A.A., Shestak, A.G., et al., Dilated cardiomyopathy: genetic causes and the strategy of DNA diagnostics, Klin. Eksp. Khir. Zh. im. Akad. B.V. Petrovskogo, 2019, vol. 7, no. 3, pp. 44—53. https://doi.org/10.24411/2308-1198-2019-13005
Hey, T.M., Rasmussen, T.B., Madsen, T., et al., Clinical and genetic investigations of 109 index patients with dilated cardiomyopathy and 445 of their relatives, Circ.: Heart Failure, 2020, vol. 13, no. 10. e006701. https://doi.org/10.1161/circheartfailure.119.006701
Haas, J., Frese, K.S., Peil, B., et al., Atlas of the clinical genetics of human dilated cardiomyopathy, Eur. Heart J., 2015, vol. 36, no. 18, pp. 1123—1135. https://doi.org/10.1093/eurheartj/ehu301
Parrott, A., Khoury, P.R., Shikany, A.R., et al., Investigation of de novo variation in pediatric cardiomyopathy, Am. J. Med. Genet., Part C., 2020, vol. 184, no. 1, pp. 116—123. https://doi.org/10.1002/ajmg.c.31764
Asselbergs, F.W., Sammani, A., Elliott, P., et al., Differences between familial and sporadic dilated cardiomyopathy: ESC EORP cardiomyopathy and myocarditis registry, ESC Heart Failure, 2021, vol. 8, no. 1, pp. 95—105. https://doi.org/10.1002/ehf2.13100
Verdonschot, J.A.J., Merken, J.J., Brunner-La Rocca, H.P., et al., Value of speckle tracking-based deformation analysis in screening relatives of patients with asymptomatic dilated cardiomyopathy, JACC Cardiovasc. Imaging, 2020, vol. 13, no. 2, part. 2, pp. 549—558. https://doi.org/10.1016/j.jcmg.2019.02.032
Paldino, A., De Angelis, G., Dal Ferro, M., et al., High prevalence of subtle systolic and diastolic dysfunction in genotype-positive phenotype-negative relatives of dilated cardiomyopathy patients, Int. J. Cardiol., 2021, vol. 324, pp. 108—114. https://doi.org/10.1016/j.ijcard.2020.09.036
Marume, K., Noguchi, T., Tateishi, E., et al., Prognosis and clinical characteristics of dilated cardiomyopathy with family history via pedigree analysis, Circ. J., 2020, vol. 84, no. 8, pp. 1284—1293. https://doi.org/10.1253/circj.CJ-19-1176
Fang, H.J. and Liu, B.P., Prevalence of TTN mutations in patients with dilated cardiomyopathy: a meta-analysis, Herz, 2020, vol. 45, suppl. 1, pp. 29—36. https://doi.org/10.1007/s00059-019-4825-4
Sivitskaya, L.N., Vaikhanskaya, T.G., Danilenko, N.G., et al., Mutations in the LMNA gene leading to conformational changes in lamins A/C and the development of dilated cardiomyopathy, Mol. Prikl. Genet., 2017, vol. 23, pp. 67—74.
Vaikhanskaya, T.G., Sivitskaya, L.N., Levdanskii, O.D., et al., Dilated cardiomyopathy: genetic predictors in risk stratification for sudden death, Kardiol. Belarusi, 2019, vol. 11, no. 4, pp. 590—602.
Kurushko, T.V., Vaikhanskaya, T.G., Bulgak, A.G., et al., LMNA-associated dilated cardiomyopathy: variability of clinical manifestations, Kardiol. Belarusi, 2018, vol. 10, no. 6, pp. 892—903.
Online Mendelian Inheritance in Man. https://omim.org/. Accessed January, 2021.
Povysil, G., Chazara, O., Carss, K.J., et al., Assessing the role of rare genetic variation in patients with heart failure, JAMA Cardiol., 2021, vol. 6, no. 4. e206500. https://doi.org/10.1001/jamacardio.2020.6500
Schultheiss, H.P., Fairweather, D., Caforio, A.L.P., et al., Dilated cardiomyopathy, Nat. Rev. Dis. Primers, 2019, vol. 5, no. 1, p. 32. https://doi.org/10.1038/s41572-019-0084-1
Hunter, L., Ferguson, R., and McDevitt, H., Vitamin D deficiency cardiomyopathy in Scotland: a retrospective review of the last decade, Arch. Dis. Child., 2020, vol. 105, no. 9, pp. 853—856. https://doi.org/10.1136/archdischild-2019-317794
Marstrand, P., Picard, K., and Lakdawala, N.K., Second hits in dilated cardiomyopathy, Curr. Cardiol. Rep., 2020, vol. 22, no. 2, p. 8. https://doi.org/10.1007/s11886-020-1260-3
Kurbanov, R.D., Abdullaev, T.A., Mirzarakhimova, S.T., and Mardanov, B.U., Peripartum cardiomyopathy: some features of clinical picture and course of the disease, Kardiologiya, 2012, vol. 52, no. 6, pp. 35—39.
Robertson, J., Lindgren, M., Schaufelberger, M., et al., Body mass index in young women and risk of cardiomyopathy: a long-term follow-up study in Sweden, Circulation, 2020, vol. 141, no. 7, pp. 520—529. https://doi.org/10.1161/circulationaha.119.044056
Peters, S., Johnson, R., Birch, S., et al., Familial dilated cardiomyopathy, Heart Lung Circ., 2020, vol. 29, no. 4, pp. 566—574. https://doi.org/10.1016/j.hlc.2019.11.018
The portal for rare diseases and orphan drugs. https://www.orpha.net/consor/cgi-bin/index.php. Accessed January, 2021.
MalaCards: the human disease database. https://www.malacards.org/. Accessed April, 2021.
ClinVar. https://www.ncbi.nlm.nih.gov/clinvar/. Accessed April, 2021.
Clinical Genome Resource: ClinGen. https://clinicalgenome.org/. Accessed April, 2021.
UniProt. https://www.uniprot.org/. Accessed April, 2021.
Protein Atlas. https://www.proteinatlas.org/. Accessed January, 2021.
Villard, E., Perret, C., Gary, F., et al., A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy, Eur. Heart J., 2011, vol. 32, no. 9, pp. 1065—1076. https://doi.org/10.1093/eurheartj/ehr105
Meder, B., Ruhle, F., Weis, T., et al., A genome-wide association study identifies 6p21 as novel risk locus for dilated cardiomyopathy, Eur. Heart J., 2014, vol. 35, no. 16, pp. 1069—1077. https://doi.org/10.1093/eurheartj/eht251
Esslinger, U., Garnier, S., Korniat, A., et al., Exome-wide association study reveals novel susceptibility genes to sporadic dilated cardiomyopathy, PLoS One, 2017, vol. 12, no. 3. e0172995. https://doi.org/10.1371/journal.pone.0172995
Vasan, R.S., Glazer, N.L., Felix, J.F., et al., Genetic variants associated with cardiac structure and function: a meta-analysis and replication of genome-wide association data, JAMA, 2009, vol. 302, no. 2, pp. 168—178. https://doi.org/10.1001/jama.2009.978-a
Wild, P.S., Felix, J.F., Schillert, A., et al., Large-scale genome-wide analysis identifies genetic variants associated with cardiac structure and function, J. Clin. Invest., 2017, vol. 127, no. 5, pp. 1798—1812. https://doi.org/10.1172/JCI84840
Aung, N., Vargas, J.D., Yang, C., et al., Genome-wide analysis of left ventricular image-derived phenotypes identifies fourteen loci associated with cardiac morphogenesis and heart failure development, Circulation, 2019, vol. 140, no. 16, pp. 1318—1330. https://doi.org/10.1161/circulationaha.119.041161
Pirruccello, J.P., Bick, A., Wang, M., et al., Analysis of cardiac magnetic resonance imaging in 36 000 individuals yields genetic insights into dilated cardiomyopathy, Nat. Commun., 2020, vol. 11, no. 1, p. 2254. https://doi.org/10.1038/s41467-020-15823-7
Alila-Fersi, O., Tabebi, M., Maalej, M., et al., First description of a novel mitochondrial mutation in the MT-TI gene associated with multiple mitochondrial DNA deletion and depletion in family with severe dilated mitochondrial cardiomyopathy, Biochem. Biophys. Res. Commun., 2018, vol. 497, no. 4, pp. 1049—1054. https://doi.org/10.1016/j.bbrc.2018.02.173
Govindaraj, P., Rani, B., Sundaravadivel, P., et al., Mitochondrial genome variations in idiopathic dilated cardiomyopathy, Mitochondrion, 2019, vol. 48, pp. 51—59. https://doi.org/10.1016/j.mito.2019.03.003
Peña-Peña, M.L., Ochoa, J.P., Barriales-Villa, R., et al., Prognostic implications of pathogenic truncating variants in the TTN gene, Int. J. Cardiol., 2020, vol. 316, pp. 180—183. https://doi.org/10.1016/j.ijcard.2020.04.086
Zhang, X.L., Xie, J., Lan, R.F., et al., Genetic basis and genotype—phenotype correlations in Han Chinese patients with idiopathic dilated cardiomyopathy, Sci. Rep., 2020, vol. 10, no. 1, p. 2226. https://doi.org/10.1038/s41598-020-58984-7
Lesurf, R., Said, A., Akinrinade, O., et al., Whole genome sequencing delineates regulatory and novel genic variants in childhood cardiomyopathy, medRxiv, 2020. https://www.medrxiv.org/. https://doi.org/10.1101/2020.10.12.20211474.
Mazzarotto, F., Tayal, U., Buchan, R.J., et al., Reevaluating the genetic contribution of monogenic dilated cardiomyopathy, Circulation, 2020, vol. 141, no. 5, pp. 387—398. https://doi.org/10.1161/circulationaha.119.037661
Herkert, J.C., Abbott, K.M., Birnie, E., et al., Toward an effective exome-based genetic testing strategy in pediatric dilated cardiomyopathy, Genet. Med., 2018, vol. 20, no. 11, pp. 1374—1386. https://doi.org/10.1038/gim.2018.9
Patterson, J., Coats, C., and McGowan, R., Familial dilated cardiomyopathy associated with pathogenic TBX5 variants: expanding the cardiac phenotype associated with Holt—Oram syndrome, Am. J. Med. Genet., Part A, 2020, vol. 182, no. 7, pp. 1725—1734. https://doi.org/10.1002/ajmg.a.61635
Rethanavelu, K., Fung, J.L.F., Chau, J.F.T., et al., Phenotypic and mutational spectrum of 21 Chinese patients with Alström syndrome, Am. J. Med. Genet., Part A, 2020, vol. 182, no. 2, pp. 279—288. https://doi.org/10.1002/ajmg.a.61412
Hawley, M.H., Almontashiri, N., and Biesecker, L., An assessment of the role of vinculin loss of function variants in inherited cardiomyopathy, Hum. Mutat., 2020, vol. 41, no. 9, pp. 1577—1587. https://doi.org/10.1002/humu.24061
Rajapreyar, I., Sinkey, R., Pamboukian, S.V., and Tita, A., Did a shared thioredoxin-reductase gene mutation lead to maternal peripartum cardiomyopathy and fatal dilated cardiomyopathy in her son? A case report, Case Rep. Womens Health, 2020, vol. 26. e00196. https://doi.org/10.1016/j.crwh.2020.e00196
Sun, Q., Guo, J., Hao, C., et al., Whole-exome sequencing reveals two de novo variants in the RBM20 gene in two Chinese patients with left ventricular non-compaction cardiomyopathy, Pediatr. Invest., 2020, vol. 4, no. 1, pp. 11—16. https://doi.org/10.1002/ped4.12183
Bukaeva, A.A., Zaklyazminskaya, E.V., Dombrovskaya, A.V., et al., Contribution of mutations in the TNNT2 gene to the spectrum of genetic causes of DCM in Russian patients, Med. Genet., 2020, vol. 19, no. 5(214), pp. 14—15. https://doi.org/10.25557/2073-7998.2020.05.14-15
Xiao, F., Wei, Q., Wu, B., et al., Clinical exome sequencing revealed that FLNC variants contribute to the early diagnosis of cardiomyopathies in infant patients, Transl. Pediatr., 2020, vol. 9, no. 1, pp. 21—33. https://doi.org/10.21037/tp.2019.12.02
Anderson, J.L., Christensen, G.B., Escobar, H., et al., Discovery of TITIN gene truncating variant mutations and 5-year outcomes in patients with nonischemic dilated cardiomyopathy, Am. J. Cardiol., 2020, vol. 137, pp. 97—102. https://doi.org/10.1016/j.amjcard.2020.09.026
Dorsch, L.M., Kuster, D.W.D., Jongbloed, J.D.H., et al., The effect of tropomyosin variants on cardiomyocyte function and structure that underlie different clinical cardiomyopathy phenotypes, Int. J. Cardiol., 2021, vol. 323, pp. 251—258. https://doi.org/10.1016/j.ijcard.2020.08.101
Qin, X., Li, P., Qu, H.Q., et al., FLNC and MYLK2 gene mutations in a Chinese family with different phenotypes of cardiomyopathy, Int. Heart J., 2021, vol. 62, no. 1, pp. 127—134. https://doi.org/10.1536/ihj.20-351
Ganapathi, M., Argyriou, L., Martínez-Azorín, F., et al., Bi-allelic missense disease-causing variants in RPL3L associate neonatal dilated cardiomyopathy with muscle-specific ribosome biogenesis, Hum. Genet., 2020, vol. 139, no. 11, pp. 1443—1454. https://doi.org/10.1007/s00439-020-02188-6
Vaikhanskaya, T.G., Sivitskaya, L.N., Danilenko, N.G., et al., Dilation of the chambers of the heart caused by mutation in the lamin gene (LMNA), Kardiologiya, 2016, vol. 56, no. 5, pp. 85—96. https://doi.org/10.18565/cardio.2016.5.85-96
Landim-Vieira, M., Johnston, J.R., Ji, W., et al., Familial dilated cardiomyopathy associated with a novel combination of compound heterozygous TNNC1 variants, Front. Physiol., 2020, vol. 10, p. 1612. https://doi.org/10.3389/fphys.2019.01612
Augusto, J.B., Eiros, R., Nakou, E., et al., Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: a comprehensive genotype-imaging phenotype study, Eur. Heart. J. Cardiovasc. Imaging, 2020, vol. 21, no. 3, pp. 326—336. https://doi.org/10.1093/ehjci/jez188
Verdonschot, J.A.J., Robinson, E.L., James, K.N., et al., Mutations in PDLIM5 are rare in dilated cardiomyopathy but are emerging as potential disease modifiers, Mol. Genet. Genomic Med., 2020, vol. 8, no. 2. e1049. https://doi.org/10.1002/mgg3.1049
Abdallah, A.M., Carlus, S.J., Al-Mazroea, A.H., et al., Digenic inheritance of LAMA4 and MYH7 mutations in patient with infantile dilated cardiomyopathy, Medicina (Kaunas), 2019, vol. 55, no. 1, p. 17. https://doi.org/10.3390/medicina55010017
Cowan, J.R., Salyer, L., Wright, N.T., et al., SOS1 gain-of-function variants in dilated cardiomyopathy, Circ. Genom. Precis. Med., 2020, vol. 13, no. 4. e002892. https://doi.org/10.1161/circgen.119.002892
van den Hoogenhof, M.M.G., Beqqali, A., Amin, A.S., et al., RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calcium handling, Circulation, 2018, vol. 138, no. 13, pp. 1330—1342. https://doi.org/10.1161/circulationaha.117.031947
Levitas, A., Muhammad, E., Zhang, Y., et al., A novel recessive mutation in SPEG causes early onset dilated cardiomyopathy, PLoS Genet., 2020, vol. 16, no. 9. e1009000. https://doi.org/10.1371/journal.pgen.1009000
Robinson, H.K., Zaklyazminskaya, E., Povolotskaya, I., et al., Biallelic variants in PPP1R13L cause paediatric dilated cardiomyopathy, Clin. Genet., 2020, vol. 98, no. 4, pp. 331—340. https://doi.org/10.1111/cge.13812
Almomani, R., Herkert, J.C., Posafalvi, A., et al., Homozygous damaging SOD2 variant causes lethal neonatal dilated cardiomyopathy, J. Med. Genet., 2020, vol. 57, no. 1, pp. 23—30. https://doi.org/10.1136/jmedgenet-2019-106330
Blagova, O., Alieva, I., Kogan, E., et al., Mixed hypertrophic and dilated phenotype of cardiomyopathy in a patient with homozygous in-frame deletion in the MyBPC3 gene treated as myocarditis for a long time, Front. Pharmacol., 2020, vol. 11, p. 579450. https://doi.org/10.3389/fphar.2020.579450
Di, R.M., Yang, C.X., Zhao, C.M., et al., Identification and functional characterization of KLF5 as a novel disease gene responsible for familial dilated cardiomyopathy, Eur. J. Med. Genet., 2020, vol. 63, no. 4, p. 103827. https://doi.org/10.1016/j.ejmg.2019.103827
Purevjav, E., Arimura, T., Augustin, S., et al., Molecular basis for clinical heterogeneity in inherited cardiomyopathies due to myopalladin mutations, Hum. Mol. Genet., 2012, vol. 21, no. 9, pp. 2039—2053. https://doi.org/10.1093/hmg/dds022
Mazzarotto, F., Hawley, M.H., Beltrami, M., et al., Systematic large-scale assessment of the genetic architecture of left ventricular noncompaction reveals diverse etiologies, Genet. Med., 2021, vol. 23, no. 5, pp. 856—864. https://doi.org/10.1038/s41436-020-01049-x
Vissing, C.R., Rasmussen, T.B., Dybro, A.M., et al., Dilated cardiomyopathy caused by truncating titin variants: long-term outcomes, arrhythmias, response to treatment and sex differences, J. Med. Genet., 2021, vol. 58, no. 12, pp. 832—841. https://doi.org/10.1136/jmedgenet-2020-107178
Tadros, R., Francis, C., Xu, X., et al., Shared genetic pathways contribute to risk of hypertrophic and dilated cardiomyopathies with opposite directions of effect, Nat. Genet., 2021, vol. 53, no. 2, pp. 128—134. https://doi.org/10.1038/s41588-020-00762-2
DisGeNET—a database of gene-disease associations. https://www.disgenet.org/. Accessed January, 2020.
Haywood, M.E., Cocciolo, A., Porter, K.F., et al., Transcriptome signature of ventricular arrhythmia in dilated cardiomyopathy reveals increased fibrosis and activated TP53, J. Mol. Cell Cardiol., 2020, vol. 139, pp. 124—134. https://doi.org/10.1016/j.yjmcc.2019.12.010
Li, M., Parker, B.L., Pearson, E., et al., Core functional nodes and sex-specific pathways in human ischaemic and dilated cardiomyopathy, Nat. Commun., 2020, vol. 11, no. 1, p. 2843. https://doi.org/10.1038/s41467-020-16584-z
Yu, J., Zeng, C., and Wang, Y., Epigenetics in dilated cardiomyopathy, Curr. Opin. Cardiol., 2019, vol. 34, no. 3, pp. 260—269. https://doi.org/10.1097/HCO.0000000000000616
Calderon-Dominguez, M., Belmonte, T., Quezada-Feijoo, M., et al., Emerging role of microRNAs in dilated cardiomyopathy: evidence regarding etiology, Transl. Res., 2020, vol. 215, pp. 86—101. https://doi.org/10.1016/j.trsl.2019.08.007
Mansueto, G., Benincasa, G., Della Mura, N., et al., Epigenetic-sensitive liquid biomarkers and personalised therapy in advanced heart failure: a focus on cell-free DNA and microRNAs, J. Clin. Pathol., 2020, vol. 73, no. 9, pp. 535—543. https://doi.org/10.1136/jclinpath-2019-206404
Haas, J., Frese, K.S., Park, Y.J., et al., Alterations in cardiac DNA methylation in human dilated cardiomyopathy, EMBO Mol. Med., 2013, vol. 5, no. 3, pp. 413—429. https://doi.org/10.1002/emmm.201201553
Koczor, C.A., Torres, R.A., Fields, E.J., et al., Thymidine kinase and mtDNA depletion in human cardiomyopathy: epigenetic and translational evidence for energy starvation, Physiol. Genomics, 2013, vol. 45, no. 14, pp. 590—596. https://doi.org/10.1152/physiolgenomics.00014.2013
Glezeva, N., Moran, B., Collier, P., et al., Targeted DNA methylation profiling of human cardiac tissue reveals novel epigenetic traits and gene deregulation across different heart failure patient subtypes, Circ.: Heart Failure, 2019, vol. 12, no. 3. e005765. https://doi.org/10.1161/circheartfailure.118.005765
Belmonte, T., Mangas, A., Calderon-Dominguez, M., et al., Peripheral microRNA panels to guide the diagnosis of familial cardiomyopathy, Transl. Res., 2020, vol. 218, pp. 1—15. https://doi.org/10.1016/j.trsl.2020.01.003
Aleshcheva, G., Pietsch, H., Escher, F., and Schultheiss, H.P., MicroRNA profiling as a novel diagnostic tool for identification of patients with inflammatory and/or virally induced cardiomyopathies, ESC Heart Failure, 2021, vol. 8, no. 1, pp. 408—422. https://doi.org/10.1002/ehf2.13090
LaRocca, T.J., Seeger, T., Prado, M., et al., Pharmacological silencing of microRNA-152 prevents pressure overload-induced heart failure, Circ.: Heart Failure, 2020, vol. 13, no. 3. e006298. https://doi.org/10.1161/circheartfailure.119.006298
Funding
This work was carried out within the framework of the state assignment of the Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by A. Kashevarova
Supplementary Information
Rights and permissions
About this article
Cite this article
Kucher, A.N., Sleptcov, A.A. & Nazarenko, M.S. Genetic Landscape of Dilated Cardiomyopathy. Russ J Genet 58, 369–383 (2022). https://doi.org/10.1134/S1022795422030085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1022795422030085