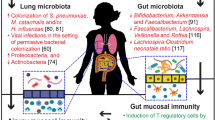
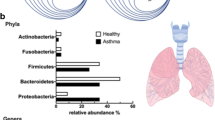
Abstract
The mucosal surfaces of the human body are typically colonized by polymicrobial communities seeded in infancy and are continuously shaped by environmental exposures. These communities interact with the mucosal immune system to maintain homeostasis in health, but perturbations in their composition and function are associated with lower airway diseases, including asthma, a developmental and heterogeneous chronic disease with various degrees and types of airway inflammation. This review will summarize recent studies examining airway microbiota dysbioses associated with asthma and their relationship with the pathophysiology of this disease.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Kuczynski J, Lauber CL, Walters WA, Parfrey LW, Clemente JC, Gevers D, et al. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet. 2012;13(1):47–58.
Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Micro. 2016;14(1):20–32. doi:10.1038/nrmicro3552.
Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324(5931):1190–2. doi:10.1126/science.1171700.
Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69(1):137–43. doi:10.1016/j.phrs.2012.11.006.
Braundmeier AG, Lenz KM, Inman KS, Chia N, Jeraldo P, Walther-António MRS, et al. Individualized medicine and the microbiome in reproductive tract. Front Physiol. 2015;6:97. doi:10.3389/fphys.2015.00097.
Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract[mdash]a role beyond infection. Nat Rev Urol. 2015;12(2):81–90. doi:10.1038/nrurol.2014.361.
Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. 2015;6(2):e00037. doi:10.1128/mBio.00037-15. Microbial migration from the oral cavity is the major source of the healthy lung microbiome.
Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med. 2011;184(8):957–63. doi:10.1164/rccm.201104-0655OC.
Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc. 2015;12(6):821–30. doi:10.1513/AnnalsATS.201501-029OC.
Koren O, Knights D, Gonzalez A, Waldron L, Segata N, Knight R, et al. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput Biol. 2013;9(1), e1002863. doi:10.1371/journal.pcbi.1002863.
Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL, et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med. 2013;187(10):1067–75. doi:10.1164/rccm.201210-1913OC.
Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Chen H, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome. 2013;1(1):1–12. doi:10.1186/2049-2618-1-19.
Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–7. doi:10.1126/science.1177486.
Lemon KP, Klepac-Ceraj V, Schiffer HK, Brodie EL, Lynch SV, Kolter R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio. 2010;1(3):e00129–10. doi:10.1128/mBio.00129-10.
Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, et al. Moving pictures of the human microbiome. Genome Biol. 2011;12(5):R50. doi:10.1186/gb-2011-12-5-r50.
The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi:10.1038/nature11234.
Oh J, Byrd AL, Deming C, Conlan S, Kong HH, Segre JA. Biogeography and individuality shape function in the human skin metagenome. Nature. 2014;514(7520):59–64. doi:10.1038/nature13786.
Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, et al. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32(8):834–41. doi:10.1038/nbt.2942.
Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–20. doi:10.1126/science.1104816.
Puertollano E, Kolida S, Yaqoob P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr Opin Clin Nutr Metab Care. 2014;17(2):139–44. doi:10.1097/mco.0000000000000025.
Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi:10.1146/annurev.nutr.22.011602.092259.
Elson CO, Alexander KL. Host-microbiota interactions in the intestine. Dig Dis. 2015;33(2):131–6. doi:10.1159/000369534.
McDermott AJ, Huffnagle GB. The microbiome and regulation of mucosal immunity. Immunology. 2014;142(1):24–31. doi:10.1111/imm.12231.
Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. doi:10.1016/j.cell.2014.03.011.
Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–93. doi:10.1016/j.cell.2012.04.037.
Tomkovich S, Jobin C. Microbiota and host immune responses: a love-hate relationship. Immunology. 2016;147(1):1–10. doi:10.1111/imm.12538.
Jain N, Walker WA. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat Rev Gastroenterol Hepatol. 2015;12(1):14–25. doi:10.1038/nrgastro.2014.153.
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6(237):237ra65–ra65. doi:10.1126/scitranslmed.3008599.
Abrahamsson TR, Wu RY, Jenmalm MC. Gut microbiota and allergy: the importance of the pregnancy period. Pediatr Res. 2015;77(1–2):214–9. doi:10.1038/pr.2014.165.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci. 2010;107(26):11971–5. doi:10.1073/pnas.1002601107.
Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD, et al. Lung microbiota promotes tolerance to allergens in neonates via PD-L1. Nat Med. 2014;20(6):642–7. doi:10.1038/nm.3568. Establishment of lung microbiota in the critical neonatal period promotes tolerance to aeroallergens.
Biesbroek G, Tsivtsivadze E, Sanders EAM, Montijn R, Veenhoven RH, Keijser BJF, et al. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190(11):1283–92. doi:10.1164/rccm.201407-1240OC. Airway microbiota succession in infancy may influence susceptibility to respiratory disease.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi:10.1038/nature12820.
Voreades N, Kozil A, Weir TL. Diet and the development of the human intestinal microbiome. Front Microbiol. 2014;5:494. doi:10.3389/fmicb.2014.00494.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. doi:10.1038/nature11053.
Petersen C, Round JL. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014;16(7):1024–33. doi:10.1111/cmi.12308.
Schulberg J, De Cruz P. Characterisation and therapeutic manipulation of the gut microbiome in inflammatory bowel disease. Intern Med J. 2016;46(3):266–73. doi:10.1111/imj.13003.
Williams MR, Gallo RL. The role of the skin microbiome in atopic dermatitis. Curr Allergy Asthma Rep. 2015;15:65. doi:10.1007/s11882-015-0567-4.
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi:10.1038/nri3785.
Sze MA, Hogg JC, Sin DD. Bacterial microbiome of lungs in COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:229–38. doi:10.2147/COPD.S38932.
Surette MG. The cystic fibrosis lung microbiome. Ann Am Thorac Soc. 2014;11 Suppl 1:S61–5. doi:10.1513/AnnalsATS.201306-159MG.
Mahdavinia M, Keshavarzian A, Tobin MC, Landay AL, Schleimer RP. A comprehensive review of the nasal microbiome in chronic rhinosinusitis (CRS). Clin Exp Allergy. 2015;46(1):21–41. doi:10.1111/cea.12666.
Huang YJ, Boushey HA. The microbiome in asthma. J Allergy Clin Immunol. 2015;135(1):25–30. doi:10.1016/j.jaci.2014.11.011.
Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6(220):220ra11–ra11. doi:10.1126/scitranslmed.3008051.
Schaubeck M, Clavel T, Calasan J, Lagkouvardos I, Haange SB, Jehmlich N, et al. Dysbiotic gut microbiota causes transmissible Crohn’s disease-like ileitis independent of failure in antimicrobial defence. Gut. 2015. doi:10.1136/gutjnl-2015-309333.
Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339(6119):548–54. doi:10.1126/science.1229000.
Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–48. doi:10.1056/NEJMra1403772.
Lundbäck B, Backman H, Lötvall J, Rönmark E. Is asthma prevalence still increasing? Expert Rev Respir Med. 2016;10(1):39–51. doi:10.1586/17476348.2016.1114417.
Loftus PA, Wise SK. Epidemiology and economic burden of asthma. Int Forum Allergy Rhinol. 2015;5:S7–10. doi:10.1002/alr.21547.
Dickson RP, Erb-Downward JR, Prescott HC, Martinez FJ, Curtis JL, Lama VN, et al. Analysis of culture-dependent versus culture-independent techniques for identification of bacteria in clinically obtained bronchoalveolar lavage fluid. J Clin Microbiol. 2014;52(10):3605–13. doi:10.1128/jcm.01028-14.
Di Bella JM, Bao Y, Gloor GB, Burton JP, Reid G. High throughput sequencing methods and analysis for microbiome research. J Microbiol Methods. 2013;95(3):401–14. doi:10.1016/j.mimet.2013.08.011.
Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol. 2013;21(7):334–41. doi:10.1016/j.tim.2013.04.002.
Vincent AT, Derome N, Boyle B, Culley AI, Charette SJ. Next-generation sequencing (NGS) in the microbiological world: how to make the most of your money. J Microbiol Methods. 2016. doi:10.1016/j.mimet.2016.02.016.
Xu L, Paterson AD, Turpin W, Xu W. Assessment and selection of competing models for zero-inflated microbiome data. PLoS One. 2015;10(7), e0129606. doi:10.1371/journal.pone.0129606.
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotech. 2013;31(9):814–21. doi:10.1038/nbt.2676.
McHardy IH, Goudarzi M, Tong M, Ruegger PM, Schwager E, Weger JR, et al. Integrative analysis of the microbiome and metabolome of the human intestinal mucosal surface reveals exquisite inter-relationships. Microbiome. 2013;1(1):17. doi:10.1186/2049-2618-1-17.
Segal LN, Clemente JC, Tsay JCJ, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol. 2016. doi:10.1038/nmicrobiol.2016.31. Enrichment of the airway microbiota with oral bacteria is associated with induction of the host mucosal immune response.
Fondi M, Liò P. Multi -omics and metabolic modelling pipelines: challenges and tools for systems microbiology. Microbiol Res. 2015;171:52–64. doi:10.1016/j.micres.2015.01.003.
Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi:10.1038/nature13568.
Turroni F, Milani C, Duranti S, Mancabelli L, Mangifesta M, Viappiani A, et al. Deciphering bifidobacterial-mediated metabolic interactions and their impact on gut microbiota by a multi-omics approach. Isme J. 2016. doi:10.1038/ismej.2015.236.
Dickson RP, Erb-Downward JR, Freeman CM, Walker N, Scales BS, Beck JM, et al. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS One. 2014;9(5), e97214. doi:10.1371/journal.pone.0097214.
Venkataraman A, Bassis CM, Beck JM, Young VB, Curtis JL, Huffnagle GB, et al. Application of a neutral community model to assess structuring of the human lung microbiome. mBio. 2015;6(1):e02284–14. doi:10.1128/mBio.02284-14.
Gleeson K, Eggli DF, Maxwell SL. Quantitative aspiration during sleep in normal subjects. Chest. 1997;111:1266–72. doi:10.1378/chest.111.5.1266.
Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126(2):410–2. doi:10.1016/j.jaci.2010.05.042. 2.e1-3.
Kembel SW, Jones E, Kline J, Northcutt D, Stenson J, Womack AM, et al. Architectural design influences the diversity and structure of the built environment microbiome. ISME J. 2012;6(8):1469–79. doi:10.1038/ismej.2011.211.
Leung MH, Wilkins D, Li EK, Kong FK, Lee PK. Indoor-air microbiome in an urban subway network: diversity and dynamics. Appl Environ Microbiol. 2014;80(21):6760–70. doi:10.1128/aem.02244-14.
Lighthart B. Mini-review of the concentration variations found in the alfresco atmospheric bacterial populations. Aerobiologia. 2000;16(1):7–16. doi:10.1023/A:1007694618888.
Dickson RP, Erb-Downward JR, Huffnagle GB. Homeostasis and its disruption in the lung microbiome. Am J Physiol Lung Cell Mol Physiol. 2015;309(10):L1047–L55. doi:10.1152/ajplung.00279.2015.
Wu W, Huang J, Duan B, Traficante DC, Hong H, Risech M, et al. Th17-stimulating protein vaccines confer protection against Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2012;186(5):420–7. doi:10.1164/rccm.201202-0182OC.
Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–73. doi:10.1183/09031936.00202013.
Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi:10.1038/nri3786.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–25. doi:10.1038/nm.2678.
Tan DJ, Walters EH, Perret JL, Lodge CJ, Lowe AJ, Matheson MC, et al. Age-of-asthma onset as a determinant of different asthma phenotypes in adults: a systematic review and meta-analysis of the literature. Expert Rev Respir Med. 2015;9(1):109–23. doi:10.1586/17476348.2015.1000311.
Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, risk factors, and mechanisms of adult-onset asthma. Mediat Inflamm. 2015;2015:514868. doi:10.1155/2015/514868.
Bhakta NR, Solberg OD, Nguyen CP, Nguyen CN, Arron JR, Fahy JV, et al. A qPCR-based metric of Th2 airway inflammation in asthma. Clin Transl Allergy. 2013;3:24. doi:10.1186/2045-7022-3-24.
Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014;133(2):388–94. doi:10.1016/j.jaci.2013.07.036.
Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104(40):15858–68. doi:10.1073/pnas.0707413104.
Woodruff PG, Modrek B, Choy DF, Jia J, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95. doi:10.1164/rccm.200903-0392OC.
Ernst G, Auteri S, Caro F, Colodenco D, Fernandez M, Menga G et al. Significant Increase of IL-8 Sputum Levels in Treatment Resistant Severe Asthma Compared with Difficult to Treat Severe Asthma Patients. J Genet Syndr Gene Ther. 2015;5(218). doi:10.4172/2157-7412.1000218
Pelaia G, Vatrella A, Busceti MT, Gallelli L, Calabrese C, Terracciano R, et al. Cellular mechanisms underlying eosinophilic and neutrophilic airway inflammation in asthma. Mediat Inflamm. 2015;2015:879783. doi:10.1155/2015/879783.
Ober C, Yao T-C. The genetics of asthma and allergic disease: a 21(st) century perspective. Immunol Rev. 2011;242(1):10–30. doi:10.1111/j.1600-065X.2011.01029.x.
Schedel M, Michel S, Gaertner VD, Toncheva AA, Depner M, Binia A, et al. Polymorphisms related to ORMDL3 are associated with asthma susceptibility, alterations in transcriptional regulation of ORMDL3, and changes in TH2 cytokine levels. J Allergy Clin Immunol. 2015;136(4):893–903. doi:10.1016/j.jaci.2015.03.014. e14.
Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol. 2013;131(1):23–30. doi:10.1016/j.jaci.2012.11.019.
Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol. 2015;15(5):308–22. doi:10.1038/nri3830.
Sevelsted A, Stokholm J, Bisgaard H. Risk of asthma from cesarean delivery depends on membrane rupture. J Pediatr. 2016. doi:10.1016/j.jpeds.2015.12.066.
Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1–2):37–47. doi:10.1016/j.cell.2014.12.020.
Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell. 2014;159(4):789–99. doi:10.1016/j.cell.2014.09.053.
Simoes CD, Maukonen J, Kaprio J, Rissanen A, Pietilainen KH, Saarela M. Habitual dietary intake is associated with stool microbiota composition in monozygotic twins. J Nutr. 2013;143(4):417–23. doi:10.3945/jn.112.166322.
Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8(1):1–16. doi:10.1186/s13073-016-0294-z.
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. doi:10.1073/pnas.1005963107.
Abrahamsson TR, Jakobsson HE, Andersson AF, Bjorksten B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy. 2014;44(6):842–50. doi:10.1111/cea.12253.
Beigelman A, Bacharier LB. Early-life respiratory infections and asthma development: role in disease pathogenesis and potential targets for disease prevention. Curr Opin Allergy Clin Immunol. 2016;16(2):172–8. doi:10.1097/aci.0000000000000244.
Busse WW, Lemanske RF, Gern JE. The role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376(9743):826–34. doi:10.1016/S0140-6736(10)61380-3.
Rantala AK, Jaakkola MS, Mäkikyrö EMS, Hugg TT, Jaakkola JJK. Early respiratory infections and the development of asthma in the first 27 years of life. Am J Epidemiol. 2015;182(7):615–23. doi:10.1093/aje/kwv093.
Fall T, Lundholm C, Ortqvist AK, Fall K, Fang F, Hedhammar A, et al. Early exposure to dogs and farm animals and the risk of childhood asthma. JAMA Pediatr. 2015;169(11), e153219. doi:10.1001/jamapediatrics.2015.3219.
Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. Jama. 2002;288(8):963–72.
Jhun I, Phipatanakul W. Early exposure to dogs and farm animals reduces risk of childhood asthma. Evid Based Med. 2016. doi:10.1136/ebmed-2015-110373.
Valkonen M, Wouters IM, Täubel M, Rintala H, Lenters V, Vasara R, et al. Bacterial exposures and associations with atopy and asthma in children. PLoS One. 2015;10(6), e0131594. doi:10.1371/journal.pone.0131594.
Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134(3):593–601. doi:10.1016/j.jaci.2014.04.018. e12.
Fujimura Kei E, Lynch SV. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe. 2015;17(5):592–602. doi:10.1016/j.chom.2015.04.007.
Bottcher MF, Bjorksten B, Gustafson S, Voor T, Jenmalm MC. Endotoxin levels in Estonian and Swedish house dust and atopy in infancy. Clin Exp Allergy. 2003;33(3):295–300.
Braun-Fahrlander C, Riedler J, Herz U, Eder W, Waser M, Grize L, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002;347(12):869–77. doi:10.1056/NEJMoa020057.
Gereda JE, Leung DY, Thatayatikom A, Streib JE, Price MR, Klinnert MD, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355(9216):1680–3.
Karvonen AM, Hyvarinen A, Gehring U, Korppi M, Doekes G, Riedler J, et al. Exposure to microbial agents in house dust and wheezing, atopic dermatitis and atopic sensitization in early childhood: a birth cohort study in rural areas. Clin Exp Allergy. 2012;42(8):1246–56. doi:10.1111/j.1365-2222.2012.04002.x.
Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349(6252):1106–10. doi:10.1126/science.aac6623. Persistent exposure to farm dust and bacterial endotoxin promotes tolerance to aeroallergens.
Khalkhali HR, Oshnouei S, Salarilak S, Rahimi Rad M, Karamyar M, Khashabi J. Effects of antibiotic consumption on children 2–8 years of age developing asthma. Epidemiol Health. 2014;36, e2014006. doi:10.4178/epih/e2014006.
Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. 2011;127(6):1125–38. doi:10.1542/peds.2010-2092.
Russell SL, Gold MJ, Hartmann M, Willing BP, Thorson L, Wlodarska M, et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13(5):440–7. doi:10.1038/embor.2012.32.
Herbst T, Sichelstiel A, Schär C, Yadava K, Bürki K, Cahenzli J, et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am J Respir Crit Care Med. 2011;184(2):198–205. doi:10.1164/rccm.201010-1574OC.
Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336(6080):489–93. doi:10.1126/science.1219328.
Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17(5):704–15. doi:10.1016/j.chom.2015.03.008. Colonization of upper airways in children with certain pathogenic bacteria is associated with acute viral respiratory infection and higher risk for asthma development.
Bisgaard H, Hermansen MN, Bonnelykke K, Stokholm J, Baty F, Skytt NL, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi:10.1136/bmj.c4978.
Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–95. doi:10.1056/NEJMoa052632.
Kloepfer KM, Lee WM, Pappas TE, Kang TJ, Vrtis RF, Evans MD, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133(5):1301–7. doi:10.1016/j.jaci.2014.02.030. e1-3 Severity of rhinovirus-induced respiratory illness and asthma exarcerbations in children are worsened by the presence of certain pathogenic bacteria.
Esposito S, Zampiero A, Terranova L, Ierardi V, Ascolese B, Daleno C, et al. Pneumococcal bacterial load colonization as a marker of mixed infection in children with alveolar community-acquired pneumonia and respiratory syncytial virus or rhinovirus infection. Pediatr Infect Dis J. 2013;32(11):1199–204. doi:10.1097/INF.0b013e31829ec274.
Grijalva CG, Griffin MR, Edwards KM, Williams JV, Gil AI, Verastegui H, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin Infect. 2014;58(10):1369–76. doi:10.1093/cid/ciu148.
Vu HT, Yoshida LM, Suzuki M, Nguyen HA, Nguyen CD, Nguyen AT, et al. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30(1):11–8. doi:10.1097/INF.0b013e3181f111a2.
Wolter N, Tempia S, Cohen C, Madhi SA, Venter M, Moyes J, et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J Infect Dis. 2014;210(10):1649–57. doi:10.1093/infdis/jiu326.
Bosch AATM, Biesbroek G, Trzcinski K, Sanders EAM, Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9(1), e1003057. doi:10.1371/journal.ppat.1003057.
Uitti JM, Tähtinen PA, Laine MK, Huovinen P, Ruuskanen O, Ruohola A. Role of nasopharyngeal bacteria and respiratory viruses in acute symptoms of young children. Pediatr Infect Dis J. 2015;34(10):1056–62. doi:10.1097/INF.0000000000000800.
Siegel SJ, Roche AM, Weiser JN. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe. 2014;16(1):55–67. doi:10.1016/j.chom.2014.06.005.
Karta MR, Gavala ML, Curran CS, Wickert LE, Keely PJ, Gern JE, et al. LPS modulates rhinovirus-induced chemokine secretion in monocytes and macrophages. Am J Respir Cell Mol Biol. 2014;51(1):125–34. doi:10.1165/rcmb.2013-0404OC.
Lee YG, Jeong JJ, Nyenhuis S, Berdyshev E, Chung S, Ranjan R, et al. Recruited alveolar macrophages, in response to airway epithelial-derived monocyte chemoattractant protein 1/CCl2, regulate airway inflammation and remodeling in allergic asthma. Am J Respir Cell Mol Biol. 2015;52(6):772–84. doi:10.1165/rcmb.2014-0255OC.
Castro-Nallar E, Shen Y, Freishtat RJ, Perez-Losada M, Manimaran S, Liu G, et al. Integrating microbial and host transcriptomics to characterize asthma-associated microbial communities. BMC Med Genet. 2015;8:50. doi:10.1186/s12920-015-0121-1.
Perez-Losada M, Castro-Nallar E, Bendall ML, Freishtat RJ, Crandall KA. Dual transcriptomic profiling of host and microbiota during health and disease in pediatric asthma. PLoS One. 2015;10(6), e0131819. doi:10.1371/journal.pone.0131819.
N’Guessan PD, Haarmann H, Steiner T, Heyl K, Schreiber F, Heinrich A, et al. The Moraxella catarrhalis-induced pro-inflammatory immune response is enhanced by the activation of the epidermal growth factor receptor in human pulmonary epithelial cells. Biochem Biophys Res Commun. 2014;450(2):1038–44. doi:10.1016/j.bbrc.2014.06.102.
Vissing NH, Larsen JM, Rasmussen MA, Chawes BL, Thysen AH, Bonnelykke K, et al. Susceptibility to lower respiratory infections in childhood is associated with perturbation of the cytokine response to pathogenic airway bacteria. Pediatr Infect Dis J. 2016. doi:10.1097/inf.0000000000001092.
Larsen JM, Brix S, Thysen AH, Birch S, Rasmussen MA, Bisgaard H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J Allergy Clin Immunol. 2014;133(4):1008–13. doi:10.1016/j.jaci.2014.01.010.
Good Jr JT, Rollins DR, Martin RJ. Macrolides in the treatment of asthma. Curr Opin Pulm Med. 2012;18(1):76–84. doi:10.1097/MCP.0b013e32834daff8.
Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10(1):67–73. doi:10.1007/s11882-009-0086-2.
Wong EHC, Porter JD, Edwards MR, Johnston SL. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2(8):657–70. doi:10.1016/S2213-2600(14)70107-9.
Bachert C, van Steen K, Zhang N, Holtappels G, Cattaert T, Maus B, et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. 2012;130(2):376–81. doi:10.1016/j.jaci.2012.05.012.
Song WJ, Chang YS, Lim MK, Yun EH, Kim SH, Kang HR, et al. Staphylococcal enterotoxin sensitization in a community-based population: a potential role in adult-onset asthma. Clin Exp Allergy. 2014;44(4):553–62. doi:10.1111/cea.12239.
Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(2):372–81. doi:10.1016/j.jaci.2010.10.048. e1-3 Composition of the bronchial airway microbiota is associated with the degree of airway hyper-responsiveness in mild asthma.
Denner DR, Sangwan N, Becker JB, Hogarth DK, Oldham J, Castillo J, et al. Corticosteroid therapy and airflow obstruction influence the bronchial microbiome, which is distinct from that of bronchoalveolar lavage in asthmatic airways. J Allergy Clin Immunol. 2015. doi:10.1016/j.jaci.2015.10.017. Composition of the bronchial airway microbiota is associated with the degree of airflow obstruction and cotricosteroid use in severe asthma.
Simpson JL, Daly J, Baines KJ, Yang IA, Upham JW, Reynolds PN, et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2015. doi:10.1183/13993003.00405-2015.
Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, et al. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136(4):874–84. doi:10.1016/j.jaci.2015.05.044.
Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1), e8578. doi:10.1371/journal.pone.0008578.
Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131(2):346–52. doi:10.1016/j.jaci.2012.11.013. e1-3.
Goleva E, Jackson LP, Kirk Harris J, Robertson CE, Sutherland ER, Hall CF, et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med. 2013;188(10):1193–201. doi:10.1164/rccm.201304-0775OC. Composition of the bronchial airway microbiota might influence responsiveness of asthmatics to treatment with corticosteroids.
Essilfie A-T, Simpson JL, Horvat JC, Preston JA, Dunkley ML, Foster PS, et al. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011;7(10), e1002244. doi:10.1371/journal.ppat.1002244.
Essilfie AT, Simpson JL, Dunkley ML, Morgan LC, Oliver BG, Gibson PG, et al. Combined Haemophilus influenzae respiratory infection and allergic airways disease drives chronic infection and features of neutrophilic asthma. Thorax. 2012;67(7):588–99. doi:10.1136/thoraxjnl-2011-200160.
Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9(6), e100645. doi:10.1371/journal.pone.0100645.
Earl CS, Keong TW, An S-q, Murdoch S, McCarthy Y, Garmendia J, et al. Haemophilus influenzae responds to glucocorticoids used in asthma therapy by modulation of biofilm formation and antibiotic resistance. EMBO Mol Med. 2015;7(8):1018–33. doi:10.15252/emmm.201505088.
Slater M, Rivett DW, Williams L, Martin M, Harrison T, Sayers I, et al. The impact of azithromycin therapy on the airway microbiota in asthma. Thorax. 2014;69(7):673–4. doi:10.1136/thoraxjnl-2013-204517.
Fairs A, Agbetile J, Hargadon B, Bourne M, Monteiro WR, Brightling CE, et al. IgE sensitization to Aspergillus fumigatus is associated with reduced lung function in asthma. Am J Respir Crit Care Med. 2010;182(11):1362–8. doi:10.1164/rccm.201001-0087OC.
Chishimba L, Niven RM, Cooley J, Denning DW. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma. 2012;49(4):423–33. doi:10.3109/02770903.2012.662568.
Denning DW, O’Driscoll BR, Powell G, Chew F, Atherton GT, Vyas A, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179(1):11–8. doi:10.1164/rccm.200805-737OC.
Urb M, Snarr BD, Wojewodka G, Lehoux M, Lee MJ, Ralph B, et al. Evolution of the immune response to chronic airway colonization with Aspergillus fumigatus Hyphae. Infect Immun. 2015;83(9):3590–600. doi:10.1128/iai.00359-15. Airway colonization with a fungal pathogen is associated with allergic inflammation and bronchial hyper-responsiveness.
Chishimba L, Fraczek MG, Niven RM, Bowyer P, Denning DW. Regulatory T-Cells/Th17 immune responses and microbiome population in severe asthma, severe asthma with fungal sensitisation (SAFS) and ABPA. Am Thorac Soc. 2015;191:A5177.
Chishimba L, Niven R, Fraczek M, Bowyer P, Smyth L, Simpson A, et al. Lung microbiome is associated with asthma severity in fungal associated asthma. Eur Respir J. 2015;46:OA1462. doi:10.1183/13993003.congress-2015.OA1462.
Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. doi:10.1126/scitranslmed.aab2271.
Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703. doi:10.1016/j.chom.2015.04.004.
Tsay TB, Yang MC, Chen PH, Hsu CM, Chen LW. Gut flora enhance bacterial clearance in lung through toll-like receptors 4. J Biomed Sci. 2011;18(1):1–8. doi:10.1186/1423-0127-18-68.
Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa EMF, Roelofs JJ, de Boer JD, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–83. doi:10.1136/gutjnl-2015-309728.
Sze MA, Tsuruta M, Yang S-WJ OY, Man SFP, Hogg JC, et al. Changes in the bacterial microbiota in gut, blood, and lungs following acute LPS instillation into mice lungs. PLoS One. 2014;9(10), e111228. doi:10.1371/journal.pone.0111228.
Verheijden KA, Willemsen LE, Braber S, Leusink-Muis T, Jeurink PV, Garssen J, et al. The development of allergic inflammation in a murine house dust mite asthma model is suppressed by synbiotic mixtures of non-digestible oligosaccharides and Bifidobacterium breve M-16V. Eur J Nutr. 2015. doi:10.1007/s00394-015-0928-8.
Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014;111(2):805–10. doi:10.1073/pnas.1. Dog-associated house dust prevents allergen-induced asthma through manipulation of the intestinal microbiome.
Pellaton C, Nutten S, Thierry AC, Boudousquie C, Barbier N, Blanchard C, et al. Intragastric and intranasal administration of Lactobacillus paracasei NCC2461 modulates allergic airway inflammation in mice. Int J Inflamm. 2012;2012:686739. doi:10.1155/2012/686739.
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–66. doi:10.1038/nm.3444. Microbial-derived short-chain fatty acids protect against allergen-induced airway inflammation.
Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics. 2013;132(3):e666–76. doi:10.1542/peds.2013-0246.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Lynch reports grants from NIH/NIAID, Janssen Inc., Broad Foundation, Sloan Foundation, Pfizer Inc., NIH/NICHD, NIH/NHLBI, and NIH/NIDDK. In addition, Dr. Lynch has a patent Stan449-PRV REDUCTIVE PRODRUG CANCER CHEMOTHERA issued, a patent Combination antibiotic and antibody therapy for the treatment of Pseudomonas aeruginosa infection with royalties paid, a patent Provisional filing for use of Lactobacillus sakei and other lactic acid bacteria as a therapeutic strategy for chronic rhinosinusitis issued, a patent Provisional filing for claims associated with use of PhyloChip as a diagnostic and prognostic clinical tool issued, and a patent Bacterial Therapeutic Consortium for induction of Immune Tolerance pending. Dr. Boushey reports grants from the National Heart, Lung, and Blood Institute and National Institute of Allergy and Infectious Diseases. Dr. Durack declares no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Asthma
Rights and permissions
About this article
Cite this article
Durack, J., Boushey, H.A. & Lynch, S.V. Airway Microbiota and the Implications of Dysbiosis in Asthma. Curr Allergy Asthma Rep 16, 52 (2016). https://doi.org/10.1007/s11882-016-0631-8
Published:
DOI: https://doi.org/10.1007/s11882-016-0631-8