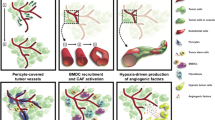
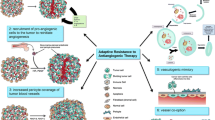
Opinion Statement
Medulloblastoma (MB) is the most frequent pediatric brain tumor. Despite conventional therapy, MB patients have high mortality and morbidity rates mainly due to the incomplete understanding of the molecular and cellular processes involved in development of this cancer. Similar to other solid tumors, MB demonstrated high endothelial cell proliferation and angiogenic activity, wherein new blood vessels arise from the pre-existing vasculature, a process named angiogenesis. MB angiogenesis is considered a hallmark for MB development, progression, and metastasis emphasizing its potential target for antitumor therapy. However, angiogenesis is tightly regulated by a set of angiogenic factors making it a complex process to be targeted. Although agents targeting these factors and their receptors are early in development, the potential for their targeting may translate into improvement in the clinical care for MB patients. In this review, we focus on the most potent angiogenic factors and their corresponding receptors, highlighting their basic properties and expression in MB. We describe their contribution to MB tumorigenesis and angiogenesis and the potential therapeutic targeting of these factors.
Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Schroeder K, Gururangan S. Molecular variants and mutations in medulloblastoma. Pharmacogenomics and personalized medicine. 2014;7:43–51.
Packer RJ, et al. Medulloblastoma: clinical and biologic aspects. Neuro Oncol. 1999;1(3):232–50.
Kiltie AE, Lashford LS, Gattamaneni HR. survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol. 1997;28(5):348–54.
Lee JW, et al. Promising survival rate but high incidence of treatment-related mortality after reduced-dose craniospinal radiotherapy and tandem high-dose chemotherapy in patients with high-risk medulloblastoma. Cancer Med. 2020;9(16):5807–18.
Louis DN, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–20.
•• Bahmad HF, Poppiti RJ. Medulloblastoma cancer stem cells: molecular signatures and therapeutic targets. J Clin Pathol. 2020;73(5):243–9 This reference is of outstanding of importance because it provided a synopsis of the novel therapeutic approaches that specifically target medulloblastoma cancer stem cells to attain enhanced anti-tumorous effects an overcome therapy resistance.
Hammoud H, et al. Drug Repurposing in Medulloblastoma: Challenges and Recommendations. Curr Treat Options Oncol. 2020;22(1):6.
Phoenix TN, et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell. 2016;29(4):508–22.
Gilhuis HJ, et al. Three-dimensional (3D) reconstruction and quantitative analysis of the microvasculature in medulloblastoma and ependymoma subtypes. Angiogenesis. 2006;9(4):201–8.
Ozer E, et al. Prognostic significance of anaplasia and angiogenesis in childhood medulloblastoma: a pediatric oncology group study. Pathol Res Pract. 2004;200(7-8):501–9.
Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–8.
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6.
• Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cellular and Molecular Life Sciences. 2020;77(9):1745–70 This reference is of importance because it discussed the current understanding of cellualr and molecular mechanisms invovled in tumor angiogenesis and highlighted challenges and opportunities associated with vascular targeting.
Huber H, et al. Angiogenic profile of childhood primitive neuroectodermal brain tumours/medulloblastomas. Eur J Cancer. 2001;37(16):2064–72.
Houck KA, et al. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5(12):1806–14.
Leung DW, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246(4935):1306–9.
Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int J Biochem Cell Biol. 2001;33(4):421–6.
Maglione D, et al. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991;88(20):9267–71.
Lyttle DJ, et al. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J Virol. 1994;68(1):84–92.
Takahashi H, et al. A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. J Biol Chem. 2004;279(44):46304–14.
Soker S, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–45.
Plate KH, et al. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359(6398):845–8.
Xu C, Wu X, Zhu J. VEGF promotes proliferation of human glioblastoma multiforme stem-like cells through VEGF receptor 2. ScientificWorldJournal. 2013;2013:417413.
Pan W, et al. TSP2 acts as a suppresser of cell invasion, migration and angiogenesis in medulloblastoma by inhibiting the Notch signaling pathway. Brain Res. 2019;1718:223–30.
Gao Y, et al. Expression levels of vascular endothelial cell growth factor and microRNA-210 are increased in medulloblastoma and metastatic medulloblastoma. Exp Ther Med. 2015;10(6):2138–44.
Pavlakovic H, et al. Quantification of angiogenesis stimulators in children with solid malignancies. Int J Cancer. 2001;92(5):756–60.
Sandén E, et al. Preoperative systemic levels of VEGFA, IL-7, IL-17A, and TNF-β delineate two distinct groups of children with brain tumors. Pediatr Blood Cancer. 2016;63(12):2112–22.
Thompson EM, et al. The role of angiogenesis in Group 3 medulloblastoma pathogenesis and survival. Neuro Oncol. 2017;19(9):1217–27.
Snuderl M, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell. 2013;152(5):1065–76.
Slongo ML, et al. Functional VEGF and VEGF receptors are expressed in human medulloblastomas. Neuro Oncol. 2007;9(4):384–92.
Wang D, et al. Microscopic delineation of medulloblastoma margins in a transgenic mouse model using a topically applied VEGFR-1 probe. Transl Oncol. 2012;5(6):408–14.
Virág J, et al. Angiogenesis and angiogenic tyrosine kinase receptor expression in pediatric brain tumors. Pathol Oncol Res. 2014;20(2):417–26.
Lin W, et al. A deregulated integrated stress response promotes interferon-γ-induced medulloblastoma. J Neurosci Res. 2011;89(10):1586–95.
Jamison S, Lin Y, Lin W. Pancreatic endoplasmic reticulum kinase activation promotes medulloblastoma cell migration and invasion through induction of vascular endothelial growth factor A. PLoS One. 2015;10(3):e0120252.
•• Korshunov A, et al. Integrated molecular analysis of adult Sonic Hedgehog (SHH)-activated medulloblastomas reveals two clinically relevant tumor subsets with VEGFA as potent prognostic indicator. Neuro Oncol. 2021;23(9):1576–85 This reference of outstanding of importance because it identified potential molecular subcategories of MBSHH with VEGFA as a potent molecular marker which may be used to improve patient stratification.
Yao X, Xie L, Zeng Y. MiR-9 promotes angiogenesis via targeting on Sphingosine-1- phosphate receptor 1. Front Cell Dev Biol. 2020;8:755.
Penco-Campillo M, et al. VEGFC negatively regulates the growth and aggressiveness of medulloblastoma cells. Commun Biol. 2020;3(1):579.
Barthomeuf C, et al. Inhibition of sphingosine-1-phosphate- and vascular endothelial growth factor-induced endothelial cell chemotaxis by red grape skin polyphenols correlates with a decrease in early platelet-activating factor synthesis. Free Radic Biol Med. 2006;40(4):581–90.
Craveiro RB, et al. In comparative analysis of multi-kinase inhibitors for targeted medulloblastoma therapy pazopanib exhibits promising in vitro and in vivo efficacy. Oncotarget. 2014;5(16):7149–61.
Ehrhardt M, et al. The FDA approved PI3K inhibitor GDC-0941 enhances in vitro the anti-neoplastic efficacy of Axitinib against c-myc-amplified high-risk medulloblastoma. J Cell Mol Med. 2018;22(4):2153–61.
Meco D, et al. Dual inhibitor AEE788 reduces tumor growth in preclinical models of medulloblastoma. Transl Oncol. 2010;3(5):326–35.
Loureiro RM, et al. ErbB2 overexpression in mammary cells upregulates VEGF through the core promoter. Biochem Biophys Res Commun. 2005;326(2):455–65.
Shi W, et al. Itraconazole side chain analogues: structure-activity relationship studies for inhibition of endothelial cell proliferation, vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, and hedgehog signaling. J Med Chem. 2011;54(20):7363–74.
Bai RY, et al. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro Oncol. 2015;17(4):545–54.
Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82.
Xian CJ. Roles of epidermal growth factor family in the regulation of postnatal somatic growth. Endocr Rev. 2007;28(3):284–96.
Zeng F, Harris RC. Epidermal growth factor, from gene organization to bedside. Seminars in cell & developmental biology. 2014;28:2–11.
Ferguson KM, et al. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11(2):507–17.
Fox SB, et al. Tumor angiogenesis in node-negative breast carcinomas—relationship with epidermal growth factor receptor, estrogen receptor, and survival. Breast Cancer Res Treat. 1994;29(1):109–16.
Goldman CK, et al. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Molecular biology of the cell. 1993;4(1):121–33.
Trojan L, et al. Expression of pro-angiogenic growth factors VEGF, EGF and bFGF and their topographical relation to neovascularisation in prostate cancer. Urol Res. 2004;32(2):97–103.
Schönholzer MT, et al. Real-time sensing of MAPK signaling in medulloblastoma cells reveals cellular evasion mechanism counteracting dasatinib blockade of ERK activation during invasion. Neoplasia. 2020;22(10):470–83.
• Maroufy V, et al. Gene expression dynamic analysis reveals co-activation of Sonic Hedgehog and epidermal growth factor followed by dynamic silencing. Oncotarget. 2020;11(15):1358–72 This reference is of importance because it provided in silico and in vito analysis of the cross talk between SHH and FGF pathways to affect the dynamic activity of gene population in MB.
Gilbertson RJ, et al. Expression of the ErbB-neuregulin signaling network during human cerebellar development: implications for the biology of medulloblastoma. Cancer Res. 1998;58(17):3932–41.
Castellino RC, et al. Heterozygosity for Pten promotes tumorigenesis in a mouse model of medulloblastoma. PLoS One. 2010;5(5):e10849.
Patereli A, et al. Expression of epidermal growth factor receptor and HER-2 in pediatric embryonal brain tumors. Pediatr Neurosurg. 2010;46(3):188–92.
Meurer RT, et al. Immunohistochemical expression of markers Ki-67, neun, synaptophysin, p53 and HER2 in medulloblastoma and its correlation with clinicopathological parameters. Arq Neuropsiquiatr. 2008;66(2b):385–90.
Das P, et al. Medulloblastomas: a correlative study of MIB-1 proliferation index along with expression of c-Myc, ERBB2, and anti-apoptotic proteins along with histological typing and clinical outcome. Childs Nerv Syst. 2009;25(7):825–35.
Liu W, et al. A prognostic analysis of pediatrics central nervous system small cell tumors: evaluation of EGFR family gene amplification and overexpression. Diagn Pathol. 2014;9:132.
Gilbertson RJ, et al. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57(15):3272–80.
Bodey B, Kaiser HE, Siegel SE. Epidermal growth factor receptor (EGFR) expression in childhood brain tumors. In Vivo. 2005;19(5):931–41.
Gilbertson R, et al. Novel ERBB4 juxtamembrane splice variants are frequently expressed in childhood medulloblastoma. Genes Chromosomes Cancer. 2001;31(3):288–94.
Korshunov A, et al. Prognostic value of tumor-associated antigens immunoreactivity and apoptosis in medulloblastomas. An analysis of 73 cases. Brain Tumor Pathol. 1999;16(1):37–44.
Min HS, et al. Medulloblastoma: histopathologic and molecular markers of anaplasia and biologic behavior. Acta Neuropathol. 2006;112(1):13–20.
Moscatello DK, et al. Frequent expression of a mutant epidermal growth factor receptor in multiple human tumors. Cancer Res. 1995;55(23):5536–9.
Rico-Varela J, et al. EGF as a new therapeutic target for medulloblastoma metastasis. Cell Mol Bioeng. 2015;8(4):553–65.
Endersby R, et al. A pre-clinical assessment of the pan-ERBB inhibitor dacomitinib in pediatric and adult brain tumors. Neoplasia. 2018;20(5):432–42.
Wolle D, et al. Inhibition of epidermal growth factor signaling by the cardiac glycoside ouabain in medulloblastoma. Cancer Med. 2014;3(5):1146–58.
Meco D, et al. Antitumor effect in medulloblastoma cells by gefitinib: Ectopic HER2 overexpression enhances gefitinib effects in vivo. Neuro Oncol. 2009;11(3):250–9.
Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207–35.
Alam R, et al. New insights into the role of fibroblast growth factors in Alzheimer's disease. Mol Biol Rep. 2021.
Abraham JA, et al. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. The EMBO journal. 1986;5(10):2523–8.
Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8(3):235–53.
Johnson DE, Williams LT. Structural and functional diversity in the FGF receptor multigene family. Adv Cancer Res. 1993;60:1–41.
•• Sarabipour S, Hristova K. Mechanism of FGF receptor dimerization and activation. Nat Commun. 2016;7:10262 This reference of outstanding of importance because it provided experimental analysis for the existence of multiple activity ligand-bound states for FGFR, and suggested a new molecular mechanism through which FGF-linked pathologies can arise.
Lamothe B, et al. The docking protein Gab1 is an essential component of an indirect mechanism for fibroblast growth factor stimulation of the phosphatidylinositol 3-kinase/Akt antiapoptotic pathway. Mol Cell Biol. 2004;24(13):5657–66.
Lee BJ, et al. Ocular biometric features of pediatric patients with fibroblast growth factor receptor-related syndromic craniosynostosis. Sci Rep. 2021;11(1):6172.
Larsson SC, Gill D. Genetic evidence supporting fibroblast growth factor 21 signalling as a pharmacological target for cardiometabolic outcomes and Alzheimer's disease. Nutrients. 2021;13(5).
Salgia R. Fibroblast growth factor signaling and inhibition in non-small cell lung cancer and their role in squamous cell tumors. Cancer Med. 2014;3(3):681–92.
Rand V, et al. Sequence survey of receptor tyrosine kinases reveals mutations in glioblastomas. Proc Natl Acad Sci U S A. 2005;102(40):14344–9.
Yu YL, et al. An acidic fibroblast growth factor protein generated by alternate splicing acts like an antagonist. J Exp Med. 1992;175(4):1073–80.
Burgess WH, et al. Possible dissociation of the heparin-binding and mitogenic activities of heparin-binding (acidic fibroblast) growth factor-1 from its receptor-binding activities by site-directed mutagenesis of a single lysine residue. J Cell Biol. 1990;111(5 Pt 1):2129–38.
Santhana Kumar K, et al. TGF-β determines the pro-migratory potential of bFGF signaling in medulloblastoma. Cell Rep. 2018;23(13):3798–3812.e8.
Brem S, et al. Immunolocalization of basic fibroblast growth factor to the microvasculature of human brain tumors. Cancer. 1992;70(11):2673–80.
• Yabut OR, et al. Aberrant FGF signaling promotes granule neuron precursor expansion in SHH subgroup infantile medulloblastoma. bioRxiv. 2021:2021.06.23.449636 This reference of importance because it identified unique and high expression of FGF5 in MBSHH patients which may be useful as a diagnostic biomarker and to test drugs specifically targeting FGF signaling in MB.
Duplan SM, Théorêt Y, Kenigsberg RL. Antitumor activity of fibroblast growth factors (FGFs) for medulloblastoma may correlate with FGF receptor expression and tumor variant. Clin Cancer Res. 2002;8(1):246–57.
Zomerman WW, et al. Exogenous HGF bypasses the effects of ErbB inhibition on tumor cell viability in medulloblastoma cell lines. PLoS One. 2015;10(10):e0141381.
Pomeroy SL, et al. Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature. 2002;415(6870):436–42.
Kenigsberg RL, et al. Effects of basic fibroblast growth factor on the differentiation, growth, and viability of a new human medulloblastoma cell line (UM-MB1). Am J Pathol. 1997;151(3):867–81.
Vachon P, Girard C, Théorêt Y. Effects of basic fibroblastic growth factor on the growth of human medulloblastoma xenografts. J Neurooncol. 2004;67(1-2):139–46.
Emmenegger BA, et al. Distinct roles for fibroblast growth factor signaling in cerebellar development and medulloblastoma. Oncogene. 2013;32(35):4181–8.
Fogarty MP, et al. Fibroblast growth factor blocks Sonic hedgehog signaling in neuronal precursors and tumor cells. Proc Natl Acad Sci U S A. 2007;104(8):2973–8.
• Holzhauser S, et al. Targeting fibroblast growth factor receptor (FGFR) and phosphoinositide 3-kinase (PI3K) signaling pathways in medulloblastoma cell lines. Anticancer Res. 2020;40(1):53–66 This reference of importance because it clearly highlighted the beneficial effect of combined treatment with FGFR and PIK3 inhibitors especially in the most resistant MB cell line.
Allen E, Walters IB, Hanahan D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17(16):5299–310.
Wang L, et al. A novel monoclonal antibody to fibroblast growth factor 2 effectively inhibits growth of hepatocellular carcinoma xenografts. Molecular cancer therapeutics. 2012;11(4):864–72.
Zhao G, et al. A novel, selective inhibitor of fibroblast growth factor receptors that shows a potent broad spectrum of antitumor activity in several tumor xenograft models. Mol Cancer Ther. 2011;10(11):2200–10.
Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15(4):197–204.
Erlandsson A, Enarsson M, Forsberg-Nilsson K. Immature neurons from CNS stem cells proliferate in response to platelet-derived growth factor. J Neurosci. 2001;21(10):3483–91.
Smits A, Ballagi AE, Funa K. PDGF-BB exerts trophic activity on cultured GABA interneurons from the newborn rat cerebellum. Eur J Neurosci. 1993;5(8):986–94.
Williams BP, et al. A PDGF-regulated immediate early gene response initiates neuronal differentiation in ventricular zone progenitor cells. Neuron. 1997;18(4):553–62.
Li WL, et al. Platelet derived growth factor receptor alpha is essential for establishing a microenvironment that supports definitive erythropoiesis. J Biochem. 2006;140(2):267–73.
Oikawa T, et al. Three isoforms of platelet-derived growth factors all have the capability to induce angiogenesis in vivo. Biol Pharm Bull. 1994;17(12):1686–8.
Risau W, et al. Platelet-derived growth factor is angiogenic in vivo. Growth Factors. 1992;7(4):261–6.
Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014;9(2):168–81.
Lange S, et al. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovascular Research. 2008;81(1):159–68.
Laschke MW, et al. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006;21(1):262–8.
Magnusson PU, et al. Platelet-derived growth factor receptor-beta constitutive activity promotes angiogenesis in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2007;27(10):2142–9.
Fujimoto K, et al. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34(9):1439–47.
Li C, et al. Microvessel density and expression of vascular endothelial growth factor, basic fibroblast growth factor, and platelet-derived endothelial growth factor in oral squamous cell carcinomas. Int J Oral Maxillofac Surg. 2005;34(5):559–65.
Saeki T, et al. Correlation between expression of platelet-derived endothelial cell growth factor (thymidine phosphorylase) and microvessel density in early-stage human colon carcinomas. Jpn J Clin Oncol. 1997;27(4):227–30.
Raica M, Cimpean AM. Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals (Basel). 2010;3(3):572–99.
Whelan HT, et al. Medulloblastoma cell line secretes platelet-derived growth factor. Pediatr Neurol. 1989;5(6):347–52.
Andrae J, et al. Platelet-derived growth factor-B and -C and active alpha-receptors in medulloblastoma cells. Biochem Biophys Res Commun. 2002;296(3):604–11.
LaRochelle WJ, et al. Platelet-derived growth factor D: tumorigenicity in mice and dysregulated expression in human cancer. Cancer Res. 2002;62(9):2468–73.
Nakamura T, et al. Expression of the B-chain of platelet-derived growth factor and proliferative activity of human brain tumors. Neurol Med Chir (Tokyo). 1993;33(4):205–11.
Faria CC, et al. Foretinib is effective therapy for metastatic sonic hedgehog medulloblastoma. Cancer Res. 2015;75(1):134–46.
Blom T, et al. Amplification and overexpression of KIT, PDGFRA, and VEGFR2 in medulloblastomas and primitive neuroectodermal tumors. J Neurooncol. 2010;97(2):217–24.
Smits A, et al. Coexpression of platelet-derived growth factor alpha and beta receptors on medulloblastomas and other primitive neuroectodermal tumors is consistent with an immature stem cell and neuronal derivation. Lab Invest. 1996;74(1):188–98.
MacDonald TJ, et al. Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease. Nat Genet. 2001;29(2):143–52.
Gilbertson RJ, Clifford SC. PDGFRB is overexpressed in metastatic medulloblastoma. Nat Genet. 2003;35(3):197–8.
Abouantoun TJ, Castellino RC, MacDonald TJ. Sunitinib induces PTEN expression and inhibits PDGFR signaling and migration of medulloblastoma cells. J Neurooncol. 2011;101(2):215–26.
Abouantoun TJ, MacDonald TJ. Imatinib blocks migration and invasion of medulloblastoma cells by concurrently inhibiting activation of platelet-derived growth factor receptor and transactivation of epidermal growth factor receptor. Mol Cancer Ther. 2009;8(5):1137–47.
Ohshima-Hosoyama S, et al. Preclinical testing of tandutinib in a transgenic medulloblastoma mouse model. J Pediatr Hematol Oncol. 2012;34(2):116–21.
Daudigeos-Dubus E, et al. Regorafenib: Antitumor Activity upon Mono and Combination Therapy in Preclinical Pediatric Malignancy Models. PLoS One. 2015;10(11):e0142612.
Tian Z, et al. Cambogin is preferentially cytotoxic to cells expressing PDGFR. PLoS One. 2011;6(6):e21370.
Wang F, et al. A microRNA-1280/JAG2 network comprises a novel biological target in high-risk medulloblastoma. Oncotarget. 2015;6(5):2709–24.
Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8(10):404–10.
Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119(4):591–600.
Boccaccio C, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391(6664):285–8.
Maroun CR, et al. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 1999;19(3):1784–99.
Ponzetto C, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77(2):261–71.
Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro-Oncology. 2005;7(4):436–51.
Birchmeier C, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–25.
Koochekpour S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997;57(23):5391–8.
Moriyama T, et al. Comparative analysis of expression of hepatocyte growth factor and its receptor, c-met, in gliomas, meningiomas and schwannomas in humans. Cancer Lett. 1998;124(2):149–55.
Rosen EM, et al. Scatter factor expression and regulation in human glial tumors. Int J Cancer. 1996;67(2):248–55.
Li Y, et al. The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res. 2005;65(20):9355–62.
Onvani S, et al. Molecular genetic analysis of the hepatocyte growth factor/MET signaling pathway in pediatric medulloblastoma. Genes Chromosomes Cancer. 2012;51(7):675–88.
Kongkham PN, et al. An epigenetic genome-wide screen identifies SPINT2 as a novel tumor suppressor gene in pediatric medulloblastoma. Cancer Res. 2008;68(23):9945–53.
Provençal M, et al. Tissue factor mediates the HGF/Met-induced anti-apoptotic pathway in DAOY medulloblastoma cells. J Neurooncol. 2010;97(3):365–72.
Provençal M, et al. c-Met activation in medulloblastoma induces tissue factor expression and activity: effects on cell migration. Carcinogenesis. 2009;30(7):1089–96.
Guessous F, et al. An orally bioavailable c-Met kinase inhibitor potently inhibits brain tumor malignancy and growth. Anticancer Agents Med Chem. 2010;10(1):28–35.
Kongkham PN, et al. Inhibition of the MET receptor tyrosine kinase as a novel therapeutic strategy in medulloblastoma. Transl Oncol. 2010;3(6):336–43.
Zhang Y, et al. Hepatocyte growth factor sensitizes brain tumors to c-MET kinase inhibition. Clin Cancer Res. 2013;19(6):1433–44.
Guessous F, et al. Cooperation between c-Met and focal adhesion kinase family members in medulloblastoma and implications for therapy. Mol Cancer Ther. 2012;11(2):288–97.
Labbé D, et al. The flavonols quercetin, kaempferol, and myricetin inhibit hepatocyte growth factor-induced medulloblastoma cell migration. J Nutr. 2009;139(4):646–52.
Ruegg C, Alghisi GC. Vascular integrins: therapeutic and imaging targets of tumor angiogenesis. Recent Results Cancer Res. 2010;180:83–101.
MacDonald TJ, et al. Preferential susceptibility of brain tumors to the antiangiogenic effects of an alpha(v) integrin antagonist. Neurosurgery. 2001;48(1):151–7.
Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48(4):549–54.
Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264(5158):569–71.
Taga T, et al. alpha v-Integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int J Cancer. 2002;98(5):690–7.
Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94(4):252–66.
Baryawno N, et al. Tumor-growth-promoting cyclooxygenase-2 prostaglandin E2 pathway provides medulloblastoma therapeutic targets. Neuro Oncol. 2008;10(5):661–74.
•• Haibe Y, et al. Resistance mechanisms to anti-angiogenic therapies in cancer. Frontiers in Oncology. 2020;10(221) This reference is of outstanding of importance because it clearly explained preclinical evidence for the various mechanisms of resistance to anti-angiogenic therapies and the novel therapeutic approaches to overcome them in cancer.
El-Kenawi AE, El-Remessy AB. Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. Br J Pharmacol. 2013;170(4):712–29.
Grotzer MA, et al. High microvessel density in primitive neuroectodermal brain tumors of childhood. Neuropediatrics. 2001;32(2):75–9.
Zhao M, et al. Bevacizumab and stereotactic radiosurgery achieved complete response for pediatric recurrent medulloblastoma. J Cancer Res Ther. 2018;14(Supplement):S789–s792.
Bonney PA, et al. Dramatic response to temozolomide, irinotecan, and bevacizumab for recurrent medulloblastoma with widespread osseous metastases. J Clin Neurosci. 2016;26:161–3.
Aguilera D, et al. Response to bevacizumab, irinotecan, and temozolomide in children with relapsed medulloblastoma: a multi-institutional experience. Child's Nervous System. 2013;29(4):589–96.
Okada K, et al. Phase I study of Bevacizumab plus irinotecan in pediatric patients with recurrent/refractory solid tumors. Japanese Journal of Clinical Oncology. 2013;43(11):1073–9.
Metts J, et al. A phase I study of irinotecan and temozolomide with bevacizumab in children with recurrent/refractory central nervous system tumors. Child's Nervous System. 2022.
Han K, et al. Bevacizumab dosing strategy in paediatric cancer patients based on population pharmacokinetic analysis with external validation. Br J Clin Pharmacol. 2016;81(1):148–60.
Fangusaro J, et al. Bevacizumab (BVZ)-associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT-11): a Pediatric Brain Tumor Consortium Study (PBTC-022). Cancer. 2013;119(23):4180–7.
Levy AS, et al. Temozolomide with irinotecan versus temozolomide, irinotecan plus bevacizumab for recurrent medulloblastoma of childhood: Report of a COG randomized Phase II screening trial. Pediatr Blood Cancer. 2021;68(8):e29031.
Piha-Paul SA, et al. Pediatric patients with refractory central nervous system tumors: experiences of a clinical trial combining bevacizumab and temsirolimus. Anticancer Res. 2014;34(4):1939–45.
Elice F, Rodeghiero F. Side effects of anti-angiogenic drugs. Thromb Res. 2012;129(Suppl 1):S50–3.
Funding
Not funded.
Author information
Authors and Affiliations
Contributions
Z. Saker and S. Nabha conceived the concept and idea of the present review. Z. Saker, M. Rizk and S. Nabha worked on the study design strategy and selected the topics to be discussed. Z. Saker, M. Rizk, and H. Bahmad did literature searches and screened titles and abstracts for relevance. Z. Saker and M. Rizk abstracted the data from the eligible full text articles, analyzed, and interpreted the data and drafted the manuscript. H. Bahmad and S. Nabha critically revised the manuscript. All authors have read and approved the final draft.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
Zahraa Saker declares that she has no conflict of interest. Mahdi Rizk declares that he has no conflict of interest. Hisham F. Bahmad declares that he has no conflict of interest. Sanaa M. Nabha declares that she has no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Saker, Z., Rizk, M., Bahmad, H.F. et al. Targeting Angiogenic Factors for the Treatment of Medulloblastoma. Curr. Treat. Options in Oncol. 23, 864–886 (2022). https://doi.org/10.1007/s11864-022-00981-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-022-00981-1