Abstract
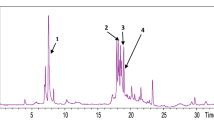
Presently, there is an increased interest in the production and purification of vegetable extracts by both pharmacological and medicinal sectors. This study aimed to optimise the phenolic extraction of corn silk and to identify phenolic compounds of the fractional extract of corn silk. Single factor experiment was used to optimise the extraction parameters. The liquid chromatography-quadrupole time-of-flight-mass spectrometer (LC-TOF/MS) system was used to identify different types of phenolic compounds in the selected fractions. The optimum conditions (i.e. extraction time of 30 min, extraction temperature of 50 °C, the solid-to-solvent ratio of 1:10 and 40% ethanol) were obtained. The corn silk was extracted using the optimum conditions and the extracted was further fractionated with hexane and ethyl acetate, subsequently. The ethyl acetate fraction exhibited the most significant free radical-scavenging activity and the highest amount of total phenolic compounds. Therefore, ethyl acetate fraction was subjected to further analysis using LC-TOF/MS. A total of 26 compounds were identified. The fractional extract was found to be rich in flavonoid compounds such as flavones, flavonols, flavanols, flavone C-glycosides, flavonols, flavonol O-glycosides, and isoflavonoids. Flavanols were the major group of flavonoids found in this fractional extract. In summary, ethyl acetate fraction of corn silk can be a good source of phenolic compounds that can be useful for application in both nutraceutical and pharmaceutical sectors.



Similar content being viewed by others
References
A. Khoddami, M.A. Wilkes, T.H. Roberts, Techniques for analysis of plant phenolic compounds. Molecules 18, 2328–2375 (2013)
D. Vauzour, K. Vafeiadou, J.P.E. Spencer, in Phytonutrients, ed. By A. Salter, H. Wiseman, G. Tucker. (Wiley-Blackwell, Chichester, 2012), pp. 110–145
D. Lamoral-Theys, L. Pottier, F. Dufrasne, J. Neve, J. Dubois, A. Kornienko, R. Kiss, L. Ingrassia, Natural polyphenols that display anticancer properties through inhibition of kinase activity. Curr. Med. Chem. 17, 812–825 (2010)
J. Dai, R.J. Mumper, Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15, 7313–7352 (2010)
A. Spatafora, C. Tringali, Natural-derived polyphenols as potential anticancer agents. Med. Chem. 12, 902–918 (2012)
M. Kampa, A.-P. Nifli, G. Notas, E. Castanas, Polyphenols and cancer cell growth. Rev. Physiol. Biochem. Pharmacol. 159, 79–113 (2007)
P. Fresco, F. Borges, M.P.M. Marques, C. Diniz, The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 16, 114–134 (2010)
Z. Bahadoran, P. Mirmiran, F. Azizi, Dietary polyphenols as potential nutraceuticals in the management of diabetes: a review. J. Diabetes Metab. Disord. 12, 43 (2013)
F.F. Anhê, Y. Desjardins, G. Pilon, S. Dudonné, M.I. Genoves, F.M. Lajol, A. Marette, Polyphenols and type 2 diabetes: a prospective review. PharmaNutrition 1, 105–114 (2013)
A.M. Ali, Anti-diabetic potential of phenolic compounds: a review. Int. J. Food Prop. 16, 91–103 (2013)
S. Habtemariam, G.K. Varghese, The antidiabetic therapeutic potential of dietary polyphenols. Curr. Pharm. Biotechnol. 15, 91–400 (2014)
R.M. van Dam, N. Naidoo, R. Landberg, Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr. Opin. Lipidol. 24, 5–33 (2013)
B. Manach, A. Mazur, A. Scalbert, Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 16, 77–84 (2005)
L.B.M. Tijburg, T. Matter, J.D. Folts, U.M. Weisgerbe, M.B. Katan, Tea flavonoids and cardiovascular diseases: a review. Crit. Rev. Food Sci. Nutr. 37, 771–785 (1997)
M. Quiñones, M. Miguel, A. Aleixandre, Beneficial effects of polyphenols on cardiovascular disease. Pharmacol. Res. 68, 125–131 (2013)
T.M. Takeuchi, C.G. Pereir, M.E.M. Braga, M.R.J. Maróstica, P.F. Leal, M.A.A. Meireles, in Extracting bioactive compounds for food products: theory and application, ed. by M.A.A. Meireles (CRC Press, Boca Raton, 2009), pp. 138–218
M.-T. Ren, J. Chen, Y. Song, L.-S. Sheng, P. Li, L.-W. Qi, Identification and quantification of 32 bioactive compounds in Lonicera species by high performance liquid chromatography coupled with time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 48, 1351–1360 (2008)
J. Guo, T. Liu, L. Han, Y. Liu, The effects of corn silk on glycaemic metabolism. Nutr. Metab. 6, 47 (2009)
Z. Maksimović, Đ Malenčić, N. Kovačević, Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 96, 873–877 (2005)
J. Liu, S. Lin, Z. Wang, C. Wang, E. Wang, Y. Zhang, J. Liu, Supercritical fluid extraction of flavonoids from Maydis stigma and its nitrite-scavenging ability. Food Bioprod. Process. 89, 333–339 (2011)
K. Hasanudin, P. Hashim, S. Mustafa, Corn silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules 17, 9697–9715 (2012)
S.W. Chan, C.Y. Lee, C.F. Yap, C.W. W.A.W.Mustapha, Ho, Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int. Food Res. J. 16, 203–213 (2009)
C. Maheshwari, M.Y. Kumar, S.K. Verma, V.K. Singh, S.N. Singh, Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol. 49, 2422–2428 (2011)
G. Miliauskas, P.R. Venskutonis, T.A.Van Beek, Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 85, 231–237 (2008)
J. Han, X. Weng, K. Bi, Antioxidants from a Chinese medicinal herb-Lithospermum erythrorhizon. Food Chem. 106, 2–10 (2008)
Y.Y. Lim, T.T. Lim, J.J. Tee, Antioxidant properties of several tropical fruits: a comparative study. Food Chem. 103, 1003–1008 (2007)
J. Jakopič, R. Veberič, Extraction of phenolic compounds from green walnut fruits in different solvents. Acta Agriculturae Slovenica 93, 11–15 (2009)
L. Tomsone, Z. Kruma, R. Galoburda, Comparison of different solvents and extraction methods for isolation of phenolic compounds from horseradish roots (Armoracia rusticana). World Acad. Sci. Eng. Technol. 64, 903–908 (2012)
I.S.C. Sulaiman, M. Basri, H.R.F. Masoumi, W.J. Chee, S.E. Ashari, M. Ismail, Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Cent. J. 11, 54 (2017)
P.W. Tan, C.P. Ta, C.W. Ho, Antioxidant properties: effects of solid-to-solvent ratio on antioxidant compounds and capacities of Pegaga (Centella asiatica). Int. Food Res. J. 18, 557–562 (2011)
D.T.P. Lien, P.T.B. Tram, H.T. Toan, Effects of extraction process on phenolic content and antioxidant activity of soybean. J. Food Nutri. Sci. 3, 33–38 (2015)
P.W. Tan, C.P. Tan, C.W. Ho, Antioxidant properties: effects of solid-to-solvent ratio on antioxidant compounds and capacities of Pegaga (Centella asiatica). Int. Food Res. J. 18, 557–562 (2011)
S. La, C.M. Sia, G.A. Akowuah, P.N. Okechukwu, H.S. Yim, The effect of extraction conditions on total phenolic content and free radical scavenging capacity of selected tropical fruits’ peel. Health Environ. J. 4, 80–102 (2013)
M. Nakamura, J.-H. Ra, Y. Jee, J.-S. Kim, Impact of different partitioned solvents on chemical composition and bioavailability of Sasa quelpaertensis Nakai leaf extract. J. Food Drug Analys. 25, 316–326 (2017)
N. Das, M.E. Islam, N. Jahan, M.S. Islam, A. Khan, M.R. Islam, M.S. Parvin, Antioxidant activities of ethanol extracts and fractions of Crescentia cujete leaves and stem bark and the involvement of phenolic compounds. BMC Complement. Altern. Med. 14, 5 (2014)
J.B. Harborne, H. Baxter, in The Handbook of Natural Flavonoids, vol. 2. ed. by J.B. By, H. Harborne, Baxter (John Wiley & Sons, Chichester, 1999)
J.P. Metabolomics, (2008). http://metabolomics.jp/wiki/Reference:Zhang_PC:Xu_SX:,Chin._Chem._Lett.,2002,13,337. Accessed 2 February 2017
M. Martínez-Vázquez, T.O.R. Apan, A.L. Lastra, R. Bye, A comparative study of the analgesic and anti-inflammatory activities of pectolinarin isolated from Cirsium subcoriaceum and linarin isolated from Buddleia cordata. Planta Med. 64, 134–137 (1998)
H. Lim, K.H. Son, H.W. Chang, K. Bae, S.S. Kang, H.P. Kim, Anti-inflammatory activity of pectolinarigenin and pectolinarin isolated from Cirsium chanroenicum. Biol. Pharm. Bull. 31, 2063–2067 (2008)
HMDB, Showing metabocard for Apigenin 7-O-(2″-O-acetylglucoside) (HMDB37341). (2017). http://www.hmdb.ca/metabolites/HMDB37341. Accessed 6 March 2017
S. Kitanaka, M. Takido, Studies on the constituents of the Leaves of Cassia torosa Cav. II. The structure of two novel flavones, Torosaflavone C and D. Chem. Pharm. Bull. 39, 3254–3257 (1991)
K. Chakrabarty, H.M. Chawla, D.K. Rastogi, Javanin, a new flavone rhamnoside from Cassia javanica immature leaves. Indian J. Chem. Sect. B. 23, 543–545 (1984)
J.L. Ingham, K.R. Markham, S.Z. Dziedzic, G.S. Pope, Puerarin 6 ″-O-β-apiofuranoside, a C-glycosylisoflavone O-glycoside from Pueraria mirifica. Phytochemistry 25, 1772–1775 (1986)
M.E. Sakalem, G. Negri, R. Tabach, Chemical composition of hydroethanolic extracts from five species of the Passiflora genus. Rev. Bras. Farmacogn. 22, 1219–1232 (2012)
G. Flamini, Flavonoids and other compounds from the aerial parts of Viola etrusca. Chem. Biodivers. 4, 139–144 (2007)
M. Kaneta, N. Sugiyama, Identification of flavone compounds in eighteen Gramineae species. Agric. Biol. Chem. 37, 2663–2665 (1973)
A. Wollenweber, J. Favre-Bonvin, M. Jay, A novel type of flavonoids: flavonol esters from fern exudates. Zeitschrift für Naturforschung C 33, 831–835 (1978)
K. Cimanga, T. De Bruyne, A. Lasure, Q. Li, L. Pieters, M. Claeys, D.V. Berghe, K. Kambu, L. Tona, A. Vlietinck, Flavonoid O-glycosides from the leaves of Morinda morindoides. Phytochemistry 38, 1301–1303 (1995)
A. Brito, J.E. Ramirez, C. Areche, B. Sepúlveda, M.J. Simirgiotis, HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 19, 17400–17421 (2014)
H. Michael, J. Salib, M. Ishak, New methoxyflavone glycosides from Verbena bipinnatifida Nutt. Die Pharmazie. 56, 348–349 (2001)
M. Leone, D. Zhai, S. Sareth, S. Kitada, J.C. Reed, M. Pellecchia, Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 63, 8118–8121 (2003)
A.L. Davis, Y. Cai, A.P. Davies, J.R. Lewis, 1H and 13C NMR assignments of some green tea polyphenols. Magn. Reson. Chem. 34, 887–890 (1996)
X. Wan, H.E. Nursten, Y. Cai, A.L. Davis, J.P.G. Wilkins, A.P. Davies, A new type of tea pigment-from the chemical oxidation of epicatechin gallate and isolated from tea. J. Sci. Food Agric. 74, 401–408 (1997)
S.J. Baek, J.-S. Kim, F.R. Jackson, T.E. Eling, M.F. McEntee, S.-H. Lee, Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis 25, 2425–2432 (2004)
Z. Zhou, C. Yang, Chemical constituents of crude green tea, the material of Pu-er tea in Yunnan. Acta Bot. Yunnanica 22, 343–350 (1999)
R. Amarowicz, F. Shahidi, Presence of two forms of methylated (−)-epigallocatechin-3-gallate in green tea. Mol. Nutri. Food Res. 47, 21–23 (2003)
Y. Fujimura, H. Tachibana, M. Maeda-Yamamoto, T. Miyase, M. Sano, K. Yamada, Antiallergic tea catechin, (−)-epigallocatechin-3-O-(3-O-methyl)-gallate, suppresses FcεRI expression in human basophilic KU812 cells. J. Agric. Food Chem. 50, 5729–5734 (2002)
M. Cheng, X. Zhang, Y. Miao, J. Cao, Z. Wu, P. Weng, The modulatory effect of (−)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3 ″Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 92, 9–16 (2017)
A. Hashimoto, G.-I. Nonaka, I. Nishioka, Tannins and related compounds. LVI. Isolation of four new acylated flavan-3-ols from oolong tea. Chem. Pharm. Bull. 35, 611–616 (1987)
N. Morita, M. Arisawa, Y. Kondo, T. Takemoto, Studies on constituents of Iris genus plants. III. The constituents of Iris florentina L. Chem. Pharm. Bull. 21, 600–603 (1973)
K. Gopinath, A. Kidwai, L. Prakash, The chemical examination of Iris nepalensis—I: structure of irisolone. Tetrahedron 16, 201–205 (1961)
J.L. Ingham, Fungal modification of pterocarpan phytoalexins from Melilotus alba and Trifolium pratense. Phytochemistry 15, 1489–1495 (1976)
T.R. Govindachari, K. Nagarajan, B.R. Pai, Chemical examination of Wedelia calendulacea. Part I. structure of wedelolactone. J. Chem. Soc. https://doi.org/10.1039/JR9560000629
S. Sarveswaran, S.C. Gautam, J. Ghosh, Wedelolactone, a medicinal plant-derived coumestan, induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCε without inhibiting Akt. Int. J. Oncol. 41, 2191–2199 (2012)
S.-C. Ren, Z.-L. Liu, X.-L. Ding, Isolation and identification of two novel flavone glycosides from corn silk (Stigma maydis). J. Med. Plants Res. 3, 1009–1015 (2009)
S. Žilić, M. Janković, Z. Basić, J. Vančetović, V. Maksimović, Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L): Comparison with medicinal herbs. J. Cereal Sci. 69, 363–370 (2016)
S. Sárosi, J. Bernáth, G. Burchi, M. Antonetti, A. Bertoli, L. Pistelli, S. Benvenuti, Effect of different plant origins and climatic conditions on the total phenolic content and total antioxidant capacity of self-heal (Prunella vulgaris L.). XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): A New Look at Medicinal and Aromatic Plants, 2010. pp. 49–55
Acknowledgements
Special thanks go to Ministry of Higher Education (MOHE) of Malaysia and Universiti Sains Malaysia. This research was supported by Grants from MOHE of Malaysia (203/PPSK/6171190).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nurraihana, H., Wan Rosli, W.I., Sabreena, S. et al. Optimisation extraction procedure and identification of phenolic compounds from fractional extract of corn silk (Zea mays hair) using LC-TOF/MS system. Food Measure 12, 1852–1862 (2018). https://doi.org/10.1007/s11694-018-9799-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9799-z